Fluorine - 9F: properties of free atoms
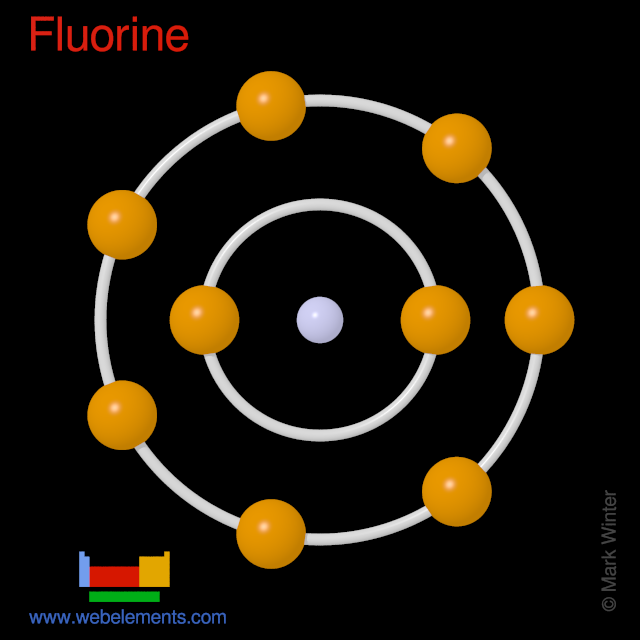
Fluorine atoms have 9 electrons and the shell structure is 2.7.
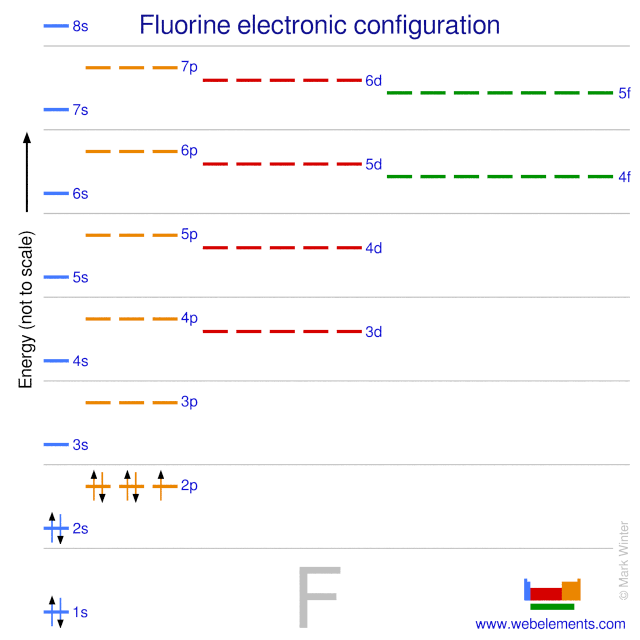
The ground state electron configuration of ground state gaseous neutral fluorine is [He].2s2.2p5 and the term symbol is 2P3/2.
Atomic spectrum
A representation of the atomic spectrum of fluorine.
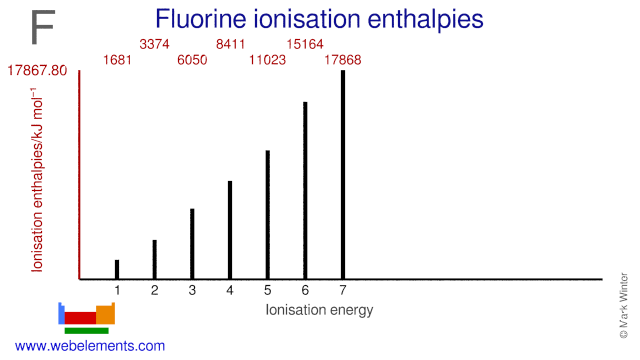
Ionisation Energies and electron affinity
The electron affinity of fluorine is 328 kJ mol‑1. The ionisation energies of fluorine are given below.
Effective Nuclear Charges
The following are "Clementi-Raimondi" effective nuclear charges, Zeff. Follow the hyperlinks for more details and for graphs in various formats.
| 1s | 8.6501 | ||||||
|---|---|---|---|---|---|---|---|
| 2s | 5.13 | 2p | 5.10 | ||||
| 3s | (no data) | 3p | (no data) | 3d | (no data) | ||
| 4s | (no data) | 4p | (no data) | 4d | (no data) | 4f | (no data) |
| 5s | (no data) | 5p | (no data) | 5d | (no data) | ||
| 6s | (no data) | 6p | (no data) | ||||
| 7s | |||||||
References
These effective nuclear charges, Zeff, are adapted from the following references:
- E. Clementi and D.L.Raimondi, J. Chem. Phys. 1963, 38, 2686.
- E. Clementi, D.L.Raimondi, and W.P. Reinhardt, J. Chem. Phys. 1967, 47, 1300.
Electron binding energies
| Label | Orbital | eV [literature reference] |
|---|---|---|
| K | 1s | 696.7 [2] |
Notes
I am grateful to Gwyn Williams (Jefferson Laboratory, Virginia, USA) who provided the electron binding energy data. The data are adapted from references 1-3. They are tabulated elsewhere on the WWW (reference 4) and in paper form (reference 5).
References
- J. A. Bearden and A. F. Burr, "Reevaluation of X-Ray Atomic Energy Levels," Rev. Mod. Phys., 1967, 39, 125.
- M. Cardona and L. Ley, Eds., Photoemission in Solids I: General Principles (Springer-Verlag, Berlin) with additional corrections, 1978.
- Gwyn Williams WWW table of values
- D.R. Lide, (Ed.) in Chemical Rubber Company handbook of chemistry and physics, CRC Press, Boca Raton, Florida, USA, 81st edition, 2000.
- J. C. Fuggle and N. Mårtensson, "Core-Level Binding Energies in Metals," J. Electron Spectrosc. Relat. Phenom., 1980, 21, 275.