Dilanthanum trisulphate nonahydrate
- Formula: La2(SO4)3.9H2O
- Hill system formula: H18La2O21S3
- CAS registry number: [10294-62-9]
- Formula weight: 728.139
- Class: complex
- Colour:
- Appearance: crystalline solid
- Melting point: 400°C (dehydrates)
- Boiling point:
- Density: 2820 kg m-3
The following are some synonyms of dilanthanum trisulphate nonahydrate:
- dilanthanum trisulphate nonahydrate
- lanthanum(III) sulphate 9-water
- dilanthanum trisulfate nonahydrate
- lanthanum sulfate 9-water
- lanthanum sulphate 9-water
- lanthanum(III) sulfate 9-water
The oxidation number of lanthanum in dilanthanum trisulphate nonahydrate is 3.
Synthesis
Not available
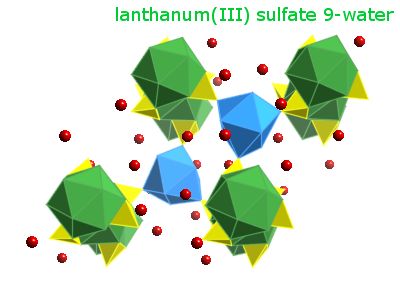
Solid state structure
- Geometry of lanthanum:
- Prototypical structure:
Element analysis
The table shows element percentages for La2(SO4)3.9H2O (dilanthanum trisulphate nonahydrate).
| Element | % |
|---|---|
| H | 2.49 |
| La | 38.15 |
| O | 46.14 |
| S | 13.21 |
Isotope pattern for La2(SO4)3.9H2O
The chart below shows the calculated isotope pattern for the formula La2(SO4)3.9H2O with the most intense ion set to 100%.
References
The data on these compounds pages are assembled and adapted from the primary literature and several other sources including the following.
- R.T. Sanderson in Chemical Periodicity, Reinhold, New York, USA, 1960.
- N.N. Greenwood and A. Earnshaw in Chemistry of the Elements, 2nd edition, Butterworth, UK, 1997.
- F.A. Cotton, G. Wilkinson, C.A. Murillo, and M. Bochmann, in Advanced Inorganic Chemistry, John Wiley & Sons, 1999.
- A.F. Trotman-Dickenson, (ed.) in Comprehensive Inorganic Chemistry, Pergamon, Oxford, UK, 1973.
- R.W.G. Wyckoff, in Crystal Structures, volume 1, Interscience, John Wiley & Sons, 1963.
- A.R.West in Basic solid state chemistry Chemistry, John Wiley & Sons, 1999.
- A.F. Wells in Structural inorganic chemistry, 4th edition, Oxford, UK, 1975.
- J.D.H. Donnay, (ed.) in Crystal data determinative tables, ACA monograph number 5, American Crystallographic Association, USA, 1963.
- D.R. Lide, (ed.) in Chemical Rubber Company handbook of chemistry and physics, CRC Press, Boca Raton, Florida, USA, 77th edition, 1996.
- J.W. Mellor in A comprehensive treatise on inorganic and theoretical chemistry, volumes 1-16, Longmans, London, UK, 1922-1937.
- J.E. Macintyre (ed.) in Dictionary of inorganic compounds, volumes 1-3, Chapman & Hall, London, UK, 1992.
