Germanium - 32Ge: the essentials
- Name: germanium
- Symbol: Ge
- Atomic number: 32
- Relative atomic mass (Ar): 72.630 (8)
- Standard state: solid at 298 K
- Appearance: greyish white
- Classification: Semi-metallic
- Group in periodic table: 14
- Group name: (none)
- Period in periodic table: 4
- Block in periodic table: p
- Shell structure: 2.8.18.4
- CAS Registry: 7440-56-4
Germanium atoms have 32 electrons and the shell structure is 2.8.18.4. The ground state electronic configuration of neutral germanium is [Ar].3d10.4s2.4p2 and the term symbol of germanium is 3P0.
Germanium: description
Germanium is a gray-white semi-metal, and in its pure state is crystalline and brittle, retaining its lustre in air at room temperature. It is a very important semiconductor material. Zone-refining techniques have led to production of crystalline germanium for semiconductor use with an impurity of only one part in 10-10.
Certain germanium compounds have a low mammalian toxicity, but a clear activity against certain bacteria, which makes them of interest as chemotherapeutic agents.

Germanium: physical properties
Density of solid: 5323 kg m-3
Molar volume: 13.63 cm3
Thermal conductivity: 60 W m‑1 K‑1
Germanium: heat properties
Melting point: 1211.4 [938.3 °C (1720.9 °F)] K
Boiling point: 3093 [2820 °C (5108 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Germanium: atom sizes
Atomic radius (empirical): 125 pm
Molecular single bond covalent radius: 121 (coordination number 4) ppm
van der Waals radius: 229 ppm
Germanium: electronegativities
Pauling electronegativity: 2.01 (Pauling units)
Allred Rochow electronegativity: 2.02 (Pauling units)
Mulliken-Jaffe electronegativity: 2.33 (sp3 orbital)
Germanium: orbital properties
First ionisation energy: 762.18 kJ mol‑1
Second ionisation energy: 1537.46 kJ mol‑1
Third ionisation energy: 3286.1 kJ mol‑1
Germanium: abundances
Universe: 200 ppb by weight
Crustal rocks: 1400 ppb by weight
Human: (no data) ppb by weight
Germanium: crystal structure
Germanium: biological data
Human abundance by weight: (no data) ppb by weight
Germanium has no biological role but is said to stimulate the metabolism.
Germanium: uses
Germanium: reactions
Reactions of germanium as the element with air, water, halogens, acids, and bases where known.
Germanium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of germanium where known.
Germanium: compound properties
Bond strengths; lattice energies of germanium halides, hydrides, oxides (where known); and reduction potentials where known.
Germanium: history
Germanium was discovered by Clemens Winkler in 1886 at Germany. Origin of name: from the Latin word "Germania" meaning "Germany".Germanium: isotopes
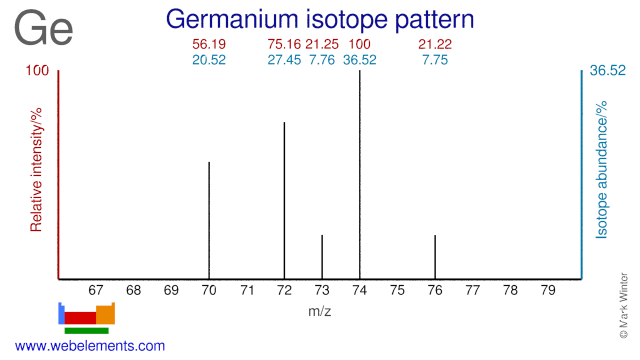
Germanium isotopes are mainly used for the production of medical As and Se radioisotopes. Ge-74 is used for the production of As-74, Ge-76 for the production of As-77, Ge-74 for the production of As-73 and Ge-72 for As-72. Ge-70, Ge-72 and Ge-74 can all be used for the production of the medical radioisotope Se-73, although the most common production route is via natural As (As-75). Natural GeF4 is used in the semiconductor pre-amorphisation implant process. The use of Ge-72, in the form of GeF4, improves this process and reduces contamination.
Germanium: isolation
Isolation: there is normally no need to make germanium in the laboratory as it is readily available commercially. Germanium is available through the treatment of germanium dioxide, GeO2, with carbon or hydrogen. The extraction of germanium from flue dust is complex because of the difficulty in separating it from zinc, which is also present.
GeO2 + 2C → Ge + 2CO
GeO2 + 2H2 → Ge + 2H2O
Very pure germanium can be made by the reaction of GeCl4 with hydrogen.
GeCl4 + 2H2 → Ge + 4HCl