Antimony - 51Sb: the essentials
- Name: antimony
- Symbol: Sb
- Atomic number: 51
- Relative atomic mass (Ar): 121.760 (1) g [see note g]
- Standard state: solid at 298 K
- Appearance: silvery lustrous grey
- Classification: Semi-metallic
- Group in periodic table: 15
- Group name: Pnictogen
- Period in periodic table: 5
- Block in periodic table: p
- Shell structure: 2.8.18.18.5
- CAS Registry: 7440-36-0
Antimony atoms have 51 electrons and the shell structure is 2.8.18.18.5. The ground state electronic configuration of neutral antimony is [Kr].4d10.5s2.5p3 and the term symbol of antimony is 4S3/2.
Antimony: description
Metallic antimony is an extremely brittle metal of a flaky, crystalline texture. It is bluish white and has a metallic lustre. It is not acted on by air at room temperature, but burns brilliantly when heated with the formation of white fumes. It is a poor conductor of heat and electricity.
Antimony and its compounds are toxic. It is found mostly with other minerals and in stibnite.

Image adapted with permission from Prof James Marshall's (U. North Texas, USA) Walking Tour of the elements CD.
Cartoon by Nick D Kim ([Science and Ink], used by permission).
Antimony: physical properties
Density of solid: 6697 kg m-3
Molar volume: 18.19 cm3
Thermal conductivity: 24 W m‑1 K‑1
Antimony: heat properties
Melting point: 903.78 [630.63 °C (1167.13 °F)] K
Boiling point: 1860 [1587 °C (2889 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Antimony: atom sizes
Atomic radius (empirical): 145 pm
Molecular single bond covalent radius: 140 (coordination number 3) ppm
van der Waals radius: 247 ppm
Antimony: electronegativities
Pauling electronegativity: 2.05 (Pauling units)
Allred Rochow electronegativity: 1.82 (Pauling units)
Mulliken-Jaffe electronegativity: 2.12 (20% s orbital)
Antimony: orbital properties
First ionisation energy: 830.58 kJ mol‑1
Second ionisation energy: 1604.2 kJ mol‑1
Third ionisation energy: 2443.35 kJ mol‑1
Antimony: abundances
Universe: 0.4 ppb by weight
Crustal rocks: 200 ppb by weight
Human: (no data) ppb by weight
Antimony: crystal structure
Antimony: biological data
Human abundance by weight: (no data) ppb by weight
Antimony has no biological role. In small doses it is said to stimulate the metabolism.
Antimony: uses
Antimony: reactions
Reactions of antimony as the element with air, water, halogens, acids, and bases where known.
Antimony: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of antimony where known.
Antimony: compound properties
Bond strengths; lattice energies of antimony halides, hydrides, oxides (where known); and reduction potentials where known.
Antimony: history
Antimony was discovered by Known since ancient times in unknown at not known. Origin of name: from the Greek words "anti + monos" meaning "not alone" (the origin of the symbol Sb comes from the Latin word "stibium").Antimony: isotopes
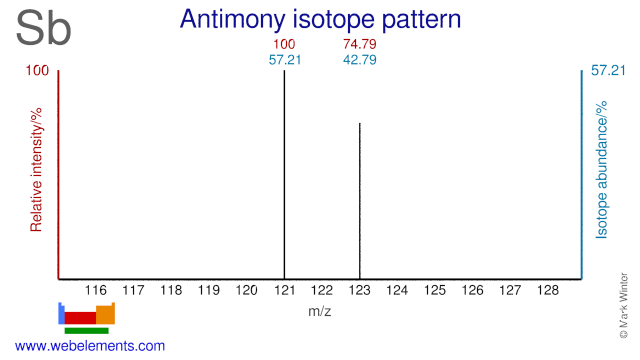
The two Antimony isotopes are used for the production of medical radioisotopes. Sb-121 can be used for the production of I-124, although this medical radioisotope is usually produced via Te-124. Sb-121 and Sb-123 can both be used for the production of I-123, although the most common production route is via Xe-124 or Te-123.
Antimony: isolation
Isolation: it is not usually necessary to make antimony in the laboratory as it is commercially available. Antimony is found in nature in a number of minerals including stibnite (Sb2S3) and ullmanite (NiSbS). Small amounts of native antimony have been found. Some ores are treatable under reducing conditions to form Sb2S3. The sulphide is removed to leave elemental antimony with scrap iron.
Sb2S3 + 3Fe → 2Sb + 3FeS
In antehr process, some ores can be heated to evolve the oxide Sb2O3 and this in turn can be reduced by charcoal in the presence of sodium sulphate, to ensure mixing, to form elemental antimony.
2Sb2O3 +3C → 4Sb + 3CO2
