Phosphorus - 15P: the essentials
- Name: phosphorus
- Symbol: P
- Atomic number: 15
- Relative atomic mass (Ar): 30.973761998 (5)
- Standard state: solid at 298 K
- Appearance: colourless/red/silvery white
- Classification: Non-metallic
- Group in periodic table: 15
- Group name: Pnictogen
- Period in periodic table: 3
- Block in periodic table: p
- Shell structure: 2.8.5
- CAS Registry: 7723-14-0
Phosphorus atoms have 15 electrons and the shell structure is 2.8.5. The ground state electronic configuration of neutral phosphorus is [Ne].3s2.3p3 and the term symbol of phosphorus is 4S3/2.
Phosphorus: description
Phosphorus is commonly misspelled "phosphorous". It is an essential component of living systems and is found in nervous tissue, bones and cell protoplasm. Phosphorus exists in several allotropic forms including white (or yellow), red, and black (or violet). White phosphorus has two modifications. Ordinary phosphorus is a waxy white solid. When pure, it is colourless and transparent. It is insoluble in water, but soluble in carbon disulphide. It catches fire spontaneously in air, burning to P4O10, often misnamed as phosphorus pentoxide. When exposed to sunlight, or when heated in its own vapour to 250°C, it is converted to the red variety. This form does not ignite spontaneously and it is a little less dangerous than white phosphorus. The red modification is fairly stable and sublimes with a vapour pressure of 1 atmosphere at 417°C.

This sample is from The Elements Collection, an attractive and safely packaged collection of the 92 naturally occurring elements that is available for sale.
Phosphorus: physical properties
Density of solid: 1823 kg m-3
Molar volume: 17.02 cm3
Thermal conductivity: 0.236 W m‑1 K‑1
Phosphorus: heat properties
Melting point: (white P) 317.3 [44.2 °C (111.6 °F)] K
Boiling point: 550 [277 °C (531 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Phosphorus: atom sizes
Atomic radius (empirical): 100 pm
Molecular single bond covalent radius: 111 (coordination number 3) ppm
van der Waals radius: 190 ppm
Phosphorus: electronegativities
Pauling electronegativity: 2.19 (Pauling units)
Allred Rochow electronegativity: 2.06 (Pauling units)
Mulliken-Jaffe electronegativity: 2.30 (20% s orbital)
Phosphorus: orbital properties
First ionisation energy: 1011.81 kJ mol‑1
Second ionisation energy: 1907.47 kJ mol‑1
Third ionisation energy: 2914.11 kJ mol‑1
Phosphorus: abundances
Universe: 7000 ppb by weight
Crustal rocks: 1000000 ppb by weight
Human: 11000000 ppb by weight
Phosphorus: crystal structure
Phosphorus: biological data
Human abundance by weight: 11000000 ppb by weight
Phosphorus is a key component of biological molecules such as DNA and RNA. Phosphorus is a component of bones, and teeth, and many other compounds required for life. Chronic poisoning of people working unprotected with white phosphorus leads to necrosis of the jaw ("phossy-jaw").
Phosphorus: uses
Phosphorus: reactions
Reactions of phosphorus as the element with air, water, halogens, acids, and bases where known.
Phosphorus: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of phosphorus where known.
Phosphorus: compound properties
Bond strengths; lattice energies of phosphorus halides, hydrides, oxides (where known); and reduction potentials where known.
Phosphorus: history
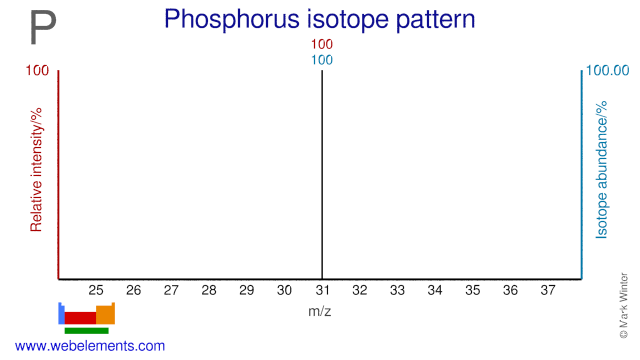
Phosphorus was discovered by Hennig Brand in 1669 at Germany. Origin of name: from the Greek word "phosphoros" meaning "bringer of light" (an ancient name for the planet Venus?).Phosphorus: isotopes
Phosphorus: isolation
Isolation: originally, phosphorus was extracted from urine. However there is plenty of phosphorus in phosphate ores and those ores represent the usual source for commercially produced phosphorus. There is normally no need to make phosphorus in the laboratory as it is readily available commercially.
The usial route involves heating a phosphate with sand and carbon in an electric furnace. It is highly energy intensive.
2Ca3(PO4)2 + 6SiO2 + 10C (1500°C) → 6CaSiO3 + 10CO + P4
The reaction may proceed via "phosphorus pentoxide", P4O10.
2Ca3(PO4)2 + 6SiO2 + → 6CaSiO3 + P4O10
P4O10 + 10C → 10CO + P4