Mercury - 80Hg: the essentials
- Name: mercury
- Symbol: Hg
- Atomic number: 80
- Relative atomic mass (Ar): 200.592 (3)
- Standard state: liquid at 298 K (the heaviest known elemental liquid)
- Appearance: silvery white
- Classification: Metallic
- Group in periodic table: 12
- Group name: (none)
- Period in periodic table: 6
- Block in periodic table: d
- Shell structure: 2.8.18.32.18.2
- CAS Registry: 7439-97-6
Mercury atoms have 80 electrons and the shell structure is 2.8.18.32.18.2. The ground state electronic configuration of neutral mercury is [Xe].4f14.5d10.6s2 and the term symbol of mercury is 1S0.
Mercury: description
Mercury is the only common metal liquid at ordinary temperatures. Mercury is sometimes called quicksilver. It rarely occurs free in nature and is found mainly in cinnabar ore (HgS) in Spain and Italy. It is a heavy, silvery-white liquid metal. It is a rather poor conductor of heat as compared with other metals but is a fair conductor of electricity. It alloys easily with many metals, such as gold, silver, and tin. These alloys are called amalgams. Its ease in amalgamating with gold is made use of in the recovery of gold from its ores.
The most important salts are mercuric chloride HgC12 (corrosive sublimate - a violent poison), mercurous chloride Hg2Cl2 (calomel, occasionally still used in medicine), mercury fulminate (Hg(ONC)2, a detonator used in explosives), and mercuric sulphide (HgS, vermillion, a high-grade paint pigment).
Organic mercury compounds are important - and dangerous. Methyl mercury is a lethal pollutant found in rivers and lakes. The main source of pollution is industrial wastes settling to the river and lake bottoms.
As mercury is a very volatile element, dangerous levels are readily attained in air. Mercury vapour should not exceed 0.1 mg m-3 in air. Air saturated with the vapour at 20°C contains mercury in a concentration far greater than that limit. The danger increases at higher temperatures. It is therefore important that mercury be handled with care. Containers of mercury should be securely covered and spillage should be avoided. Mercury should only be handled under in a well-ventilated area. If you are in possession of any mercury you are advised to contact a properly qualified chemist or public health laboratory for its safe disposal.
Small amounts of mercury spillage can be cleaned up by addition of sulphur powder. The resulting mixture should be disposed of carefully.
Cartoon by Nick D Kim ([Science and Ink], used by permission).

Mercury: physical properties
Density of solid: 14190 kg m-3
Molar volume: 14.09 cm3
Thermal conductivity: 8.3 W m‑1 K‑1
Mercury: heat properties
Melting point: 234.32 [‑38.83 °C (‑37.89 °F)] K
Boiling point: 629.88 [356.73 °C (674.11 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Mercury: atom sizes
Atomic radius (empirical): 150 pm
Molecular single bond covalent radius: 133 (coordination number 1) ppm
van der Waals radius: 247 ppm
Mercury: electronegativities
Pauling electronegativity: 2.00 (Pauling units)
Allred Rochow electronegativity: 1.44 (Pauling units)
Mulliken-Jaffe electronegativity: 1.81 (sp orbital)
Mercury: orbital properties
First ionisation energy: 1007.07 kJ mol‑1
Second ionisation energy: 1809.76 kJ mol‑1
Third ionisation energy: 3325 kJ mol‑1
Mercury: abundances
Universe: 1 ppb by weight
Crustal rocks: 67 ppb by weight
Human: (no data) ppb by weight
Mercury: crystal structure
Mercury: biological data
Human abundance by weight: (no data) ppb by weight
Mercury has no biological role but is widespread in the biosphere and in food chains, including ours.
Mercury: uses
Mercury: reactions
Reactions of mercury as the element with air, water, halogens, acids, and bases where known.
Mercury: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of mercury where known.
Mercury: compound properties
Bond strengths; lattice energies of mercury halides, hydrides, oxides (where known); and reduction potentials where known.
Mercury: history
Mercury was discovered by known since ancient times in unknown at not known. Origin of name: named after the planet "Mercury" (the origin of the symbol Hg is the Latin word "hydrargyrum" meaning "liquid silver").Mercury: isotopes
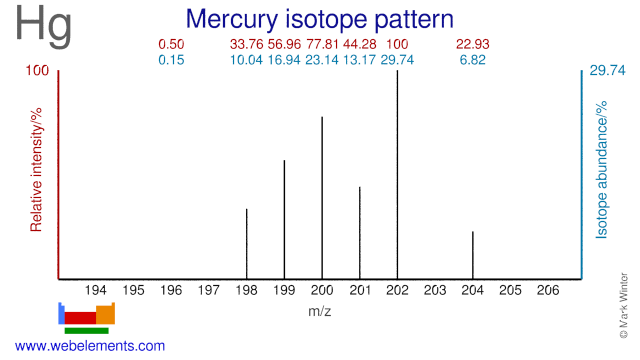
- Mercury isotopes are mainly used in the study of the deposition and emission of Hg in both terrestrial and aquatic ecosystems. In one experiment in Canada three different Hg isotopes (Hg-198, Hg-200 and Hg-202) were used to find out how the route of entry of mercury to an ecosystem affects the amount that becomes accumulated in fish. Several other trials using Hg isotopes are, or have been, undertaken in lakes in the US and Canada. Hg-202 is also used for the production of radioactive Hg-203 which is used for gamma radiation calibration.
Mercury: isolation
Isolation: the physical appearance of mercury is well known because of its use in many thermometers. It was common to demonstrate the formation of mercury in the laboratory by heating mercury sulphide (cinnabar, HgS) but this is strongly discouraged today because of the toxicity of mercury vapours. Don't do it! However, this method forms the basis of commercial extraction. The prepared cinnabar ore is heated in a current of air and the mercury vapour condensed.
HgS + O2 (600°C) → Hg (l) + SO2 (g)
The crude mercury is then washed with nitric acid and treated with air in order to remove impurities as oxides or into solution. Further purification is achieved by distillation at reduced pressure.