Silver - 47Ag: the essentials
- Name: silver
- Symbol: Ag
- Atomic number: 47
- Relative atomic mass (Ar): 107.8682 (2) g [see note g]
- Standard state: solid at 298 K
- Appearance: silver
- Classification: Metallic
- Group in periodic table: 11
- Group name: Coinage metal
- Period in periodic table: 5
- Block in periodic table: d
- Shell structure: 2.8.18.18.1
- CAS Registry: 7440-22-4
Silver atoms have 47 electrons and the shell structure is 2.8.18.18.1. The ground state electronic configuration of neutral silver is [Kr].4d10.5s1 and the term symbol of silver is 2S1/2.
Silver: description
Silver is somewhat rare and expensive, although not as expensive as gold. Slag dumps in Asia Minor and on islands in the Aegean Sea indicate that man learned to separate silver from lead as early as 3000 B.C. Pure silver has a brilliant white metallic lustre. It is a little harder than gold and is very ductile and malleable. Pure silver has the highest electrical and thermal conductivity of all metals, and possesses the lowest contact resistance. Silver iodide, AgI, is (or was?) used for causing clouds to produce rain.
Silver is stable in pure air and water, but tarnishes when exposed to ozone, hydrogen sulphide, or air containing sulphur. It occurs in ores including argentite, lead, lead-zinc, copper and gold found in Mexico, Peru, and the USA.
Cartoon by Nick D Kim ([Science and Ink], used by permission).
Silver: physical properties
Density of solid: 10490 kg m-3
Molar volume: 10.27 cm3
Thermal conductivity: 430 W m‑1 K‑1
Silver: heat properties
Melting point: 1234.93 [961.78 °C (1763.2 °F)] K
Boiling point: 2435 [2162 °C (3924 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Silver: atom sizes
Atomic radius (empirical): 160 pm
Molecular single bond covalent radius: 128 (coordination number 2) ppm
van der Waals radius: 253 ppm
Silver: electronegativities
Pauling electronegativity: 1.93 (Pauling units)
Allred Rochow electronegativity: 1.42 (Pauling units)
Mulliken-Jaffe electronegativity: 1.47 (s orbital)
Silver: orbital properties
First ionisation energy: 731.00 kJ mol‑1
Second ionisation energy: 2072.93 kJ mol‑1
Third ionisation energy: 3358 kJ mol‑1
Silver: abundances
Universe: 0.6 ppb by weight
Crustal rocks: 80 ppb by weight
Human: (no data) ppb by weight
Silver: crystal structure
Silver: biological data
Human abundance by weight: (no data) ppb by weight
Silver has no biological role.
Silver: uses
Silver: reactions
Reactions of silver as the element with air, water, halogens, acids, and bases where known.
Silver: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of silver where known.
Silver: compound properties
Bond strengths; lattice energies of silver halides, hydrides, oxides (where known); and reduction potentials where known.
Silver: history
Silver was discovered by Known since ancient times in unknown at not known. Origin of name: from the Anglo-Saxon word "siolfur" meaning "silver" (the origin of the symbol Ag comes from the Latin word "argentum" meaning "silver").Silver: isotopes
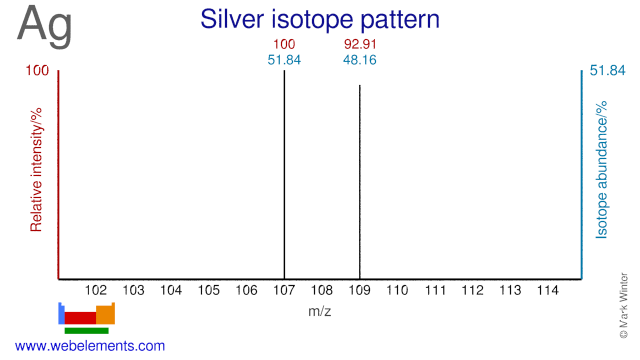
The two isotopes of Silver, Ag-107 and Ag-109 are used and have been proposed as precursor for the production of a number of radioisotopes. Ag-107 has been proposed for the (cyclotron) production of Pd-103, although the most common route for Pd-103 is via Rh-103 or Pd-104. Ag-109 is used for the production of Ag-110m which is used as a gamma reference source. Ag-109 can also be used for the production of In-110 (a replacement for the more commonly used In-111) and for the production of Cd-109, an 88 keV gamma reference source.
Silver: isolation
Isolation: silver is readily available commercially so it is not normally necessary to prepare silver in the laboratory. However the formation of silver metal may be demonstrated in a satisfying reaction in which copper metal is dipped into a solution of silver nitrate, AgNO3.
Cu(s) + 2 AgNO3 (aq) → Cu(NO3)2 + 2 Ag (s)
The result is formation of often attractive silver crystals and a blue-green solution of copper nitrate. Industrially, silver is usually a byproduct of processes whose main object is the extraction of another metal such as copper, lead, and zinc. So called "anode slimes" from the electrolytic purification of copper contain silver and a somewhat involved process is finished by an electrolysis of a nitrate solution containing silver.