Lead - 82Pb: the essentials
- Name: lead
- Symbol: Pb
- Atomic number: 82
- Relative atomic mass (Ar): 207.2 (1) g, r [see notes g m]
- Standard state: solid at 298 K
- Appearance: bluish white
- Classification: Metallic
- Group in periodic table: 14
- Group name: (none)
- Period in periodic table: 6
- Block in periodic table: p
- Shell structure: 2.8.18.32.18.4
- CAS Registry: 7439-92-1
Lead atoms have 82 electrons and the shell structure is 2.8.18.32.18.4. The ground state electronic configuration of neutral lead is [Xe].4f14.5d10.6s2.6p2 and the term symbol of lead is 3P0.
Lead: description
Lead is a bluish-white lustrous metal. It is very soft, highly malleable, ductile, and a relatively poor conductor of electricity. It is very resistant to corrosion but tarnishes upon exposure to air. Lead pipes bearing the insignia of Roman emperors, used as drains from the baths, are still in service. Alloys include pewter and solder. Tetraethyl lead (PbEt4) is still used in some grades of petrol (gasoline) but is being phased out on environmental grounds.
Lead isotopes are the end products of each of the three series of naturally occurring radioactive elements.

Cartoon by Nick D Kim ([Science and Ink], used by permission).
Lead: physical properties
Density of solid: 11340 kg m-3
Molar volume: 18.26 cm3
Thermal conductivity: 35 W m‑1 K‑1
Lead: heat properties
Melting point: 600.61 [327.46 °C (621.43 °F)] K
Boiling point: 2022 [1749 °C (3180 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Lead: atom sizes
Atomic radius (empirical): 180 pm
Molecular single bond covalent radius: 144 (coordination number 4) ppm
van der Waals radius: 254 ppm
Lead: electronegativities
Pauling electronegativity: 2.33 (Pauling units)
Allred Rochow electronegativity: 1.55 (Pauling units)
Mulliken-Jaffe electronegativity: 2.41 (sp3 orbital)
Lead: orbital properties
First ionisation energy: 715.60 kJ mol‑1
Second ionisation energy: 1450.42 kJ mol‑1
Third ionisation energy: 3081.48 kJ mol‑1
Lead: abundances
Universe: 10 ppb by weight
Crustal rocks: 10000 ppb by weight
Human: 1700 ppb by weight
Lead: crystal structure
Lead: biological data
Human abundance by weight: 1700 ppb by weight
Lead has no biological role. Lead affects the gut, central nervous system and causes anaemia.
Lead: uses
Lead: reactions
Reactions of lead as the element with air, water, halogens, acids, and bases where known.
Lead: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of lead where known.
Lead: compound properties
Bond strengths; lattice energies of lead halides, hydrides, oxides (where known); and reduction potentials where known.
Lead: history
Lead was discovered by known since ancient times in unknown at not known. Origin of name: from the Anglo-Saxon word "lead; Latin, plumbum" (the origin of the symbol Pb is the Latin word "plumbum" meaning "liquid silver".Lead: isotopes
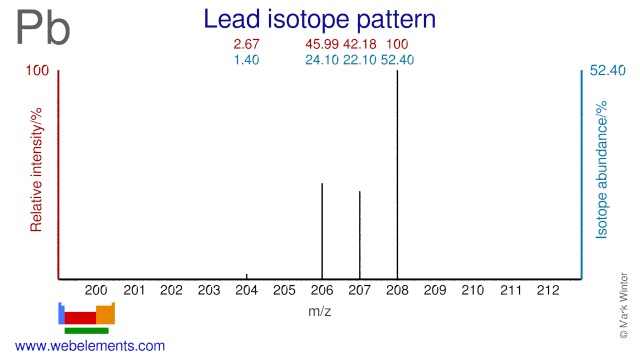
Lead isotopes are used for medical and scientific purposes. Pb-206 and Pb-207 can both be used to produce the medical radioisotopes Bi-205 and Bi-206. Pb-204, Pb-206 and Pb-207 are used to measure lead levels in blood. Pb-208 has been used to produce neutron-rich isotopes of W and Lu. Pb-208 has also been used to study the configuration of neutron stars. Several Lead isotopes have also been used as target in the production of super heavy elements.
Lead: isolation
Isolation: there is usually little need to make lead metal in the laboratory as it is so cheap and readily available. Lead is isolated from the sulphide, PbS. The process involves burning in a restricted air flow followed by reduction of the resulting oxide PbO with carbon.
PbS + 3/2O2 → PbO + SO2
PbO + C → Pb + CO
PbO + CO → Pb + CO2
This gives lead usually contaminated with metals such as antimony, arsenic, copper, gold, silver, tin, and zinc. A fairly complex process is used to strip out these impurities.