Fluorine - 9F: the essentials
- Name: fluorine
- Symbol: F
- Atomic number: 9
- Relative atomic mass (Ar): 18.998403163 (6)
- Standard state: gas at 298 K
- Appearance: pale yellow
- Classification: Non-metallic
- Group in periodic table: 17
- Group name: Halogen
- Period in periodic table: 2
- Block in periodic table: p
- Shell structure: 2.7
- CAS Registry: 7782-41-4
Fluorine atoms have 9 electrons and the shell structure is 2.7. The ground state electronic configuration of neutral fluorine is [He].2s2.2p5 and the term symbol of fluorine is 2P3/2.
Fluorine: description
Fluorine is a Group 17 element. Fluorine is the most electronegative and reactive of all elements. It is a pale yellow, corrosive gas, which reacts with practically all organic and inorganic substances. Finely divided metals, glass, ceramics, carbon, and even water burn in fluorine with a bright flame. It is not uncommon to see fluorine spelled incorrectly as flourine.
Until World War 2, there was no commercial production of elemental fluorine. Atom bomb projects and nuclear energy applications made it necessary to produce large quantities of fluorine since isotopes of uranium can be separated through the gas diffusion of UF6. Reasonably safe handling techniques for fluorine are now available and one can transport liquid fluorine by the ton. Compounds of fluorine with noble gases such as xenon, radon, and krypton are known. Elemental fluorine and the fluoride ion (in quantity) are highly toxic.
Cartoon by Nick D Kim ([Science and Ink], used by permission).
Fluorine: physical properties
Density of solid: 1700 kg m-3
Molar volume: 11.20 cm3
Thermal conductivity: 0.0277 W m‑1 K‑1
Fluorine: heat properties
Melting point: 53.53 [‑219.62 °C (‑363.32 °F)] K
Boiling point: 85.03 [‑188.12 °C (‑306.62 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Fluorine: atom sizes
Atomic radius (empirical): 50 pm
Molecular single bond covalent radius: 64 (coordination number 1) ppm
van der Waals radius: 146 ppm
Fluorine: electronegativities
Pauling electronegativity: 3.98 (Pauling units)
Allred Rochow electronegativity: 4.10 (Pauling units)
Mulliken-Jaffe electronegativity: 3.91 (14.3% s orbital)
Fluorine: orbital properties
First ionisation energy: 1681.05 kJ mol‑1
Second ionisation energy: 3374.17 kJ mol‑1
Third ionisation energy: 6050.40 kJ mol‑1
Fluorine: abundances
Universe: 400 ppb by weight
Crustal rocks: 540000 ppb by weight
Human: 37000 ppb by weight
Fluorine: crystal structure
Fluorine: biological data
Human abundance by weight: 37000 ppb by weight
Fluorine as fluoride (F-) is probably an essential element for humans and certainly is for some molluscs. In some areas, fluoride ion is added to drinking water (in very low concentrations) since it renders tooth enamel relatively immune to bacteriological attack. It does this by replacing the OH group of hydroxyapatite with fluoride. In other areas, fluoride is not added to water, despite the benefits, as a consequence of protests from civil rights activists who object to the addition of anything to water.
Fluorine: uses
Fluorine: reactions
Reactions of fluorine as the element with air, water, halogens, acids, and bases where known.
Fluorine: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of fluorine where known.
Fluorine: compound properties
Bond strengths; lattice energies of fluorine halides, hydrides, oxides (where known); and reduction potentials where known.
Fluorine: history
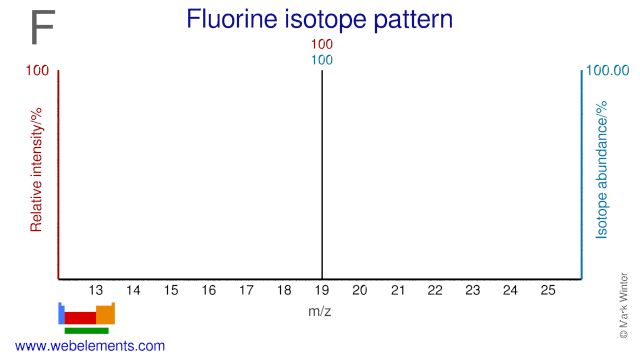
Fluorine was discovered by Henri Moissan in 1886 at France. Origin of name: from the Latin word "fluere" meaning "to flow".Fluorine: isotopes
Fluorine: isolation
Isolation: it would never be necessary to make fluorine gas in most laboratories. Fluorine is available commercially in cylinders but is very difficult to handle. Fluorine may be recovered with difficulty as a highly reactive and corrosive pale yellow gas by electrolysis of hot molten mixtures (1:2) of potassium fluoride (KF) and hydrogen fluoride (HF). The electrolyte is corrosive, so is the product. Grease must be avoided because of the fire hazard. It is difficult to store as it reacts with most materials but steel and Monel metal containers may be used as the metal surfaces deactivate through the formation of unreactive surface fluorides.