Tellurium - 52Te: the essentials
- Name: tellurium
- Symbol: Te
- Atomic number: 52
- Relative atomic mass (Ar): 127.60 (3) g [see note g]
- Standard state: solid at 298 K
- Appearance: silvery lustrous grey
- Classification: Semi-metallic
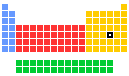
- Group in periodic table: 16
- Group name: Chalcogen
- Period in periodic table: 5
- Block in periodic table: p
- Shell structure: 2.8.18.18.6
- CAS Registry: 13494-80-9
Tellurium atoms have 52 electrons and the shell structure is 2.8.18.18.6. The ground state electronic configuration of neutral tellurium is [Kr].4d10.5s2.5p4 and the term symbol of tellurium is 3P2.
Tellurium: description
Crystalline tellurium has a silvery-white appearance, and exhibits a metallic lustre when pure (see above). It is brittle and easily pulverised. Tellurium is a p-type semiconductor, and shows varying conductivity with crystal alignment. Its conductivity increases slightly with exposure to light. It can be doped with silver, copper, gold, tin, or other elements.
Humans exposed to as little as 0.01 mg m-3 in air, or less, develop "tellurium breath", which has a garlic-like odour.

Tellurium: physical properties
Density of solid: 6240 kg m-3
Molar volume: 20.46 cm3
Thermal conductivity: 3 W m‑1 K‑1
Tellurium: heat properties
Melting point: 722.66 [449.51 °C (841.12 °F)] K
Boiling point: 1261 [988 °C (1810 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Tellurium: atom sizes
Atomic radius (empirical): 140 pm
Molecular single bond covalent radius: 136 (coordination number 2) ppm
van der Waals radius: 199 ppm
Tellurium: electronegativities
Pauling electronegativity: 2.1 (Pauling units)
Allred Rochow electronegativity: 2.01 (Pauling units)
Mulliken-Jaffe electronegativity: 2.41 (16.7% s orbital)
Tellurium: orbital properties
First ionisation energy: 869.30 kJ mol‑1
Second ionisation energy: 1795 kJ mol‑1
Third ionisation energy: 2686 kJ mol‑1
Tellurium: abundances
Universe: 9 ppb by weight
Crustal rocks: 1.0 ppb by weight
Human: (no data) ppb by weight
Tellurium: crystal structure
Tellurium: biological data
Human abundance by weight: (no data) ppb by weight
Tellurium has no biological role. All tellurium compounds are highly toxic.
Tellurium: uses
Tellurium: reactions
Reactions of tellurium as the element with air, water, halogens, acids, and bases where known.
Tellurium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of tellurium where known.
Tellurium: compound properties
Bond strengths; lattice energies of tellurium halides, hydrides, oxides (where known); and reduction potentials where known.
Tellurium: history
Tellurium was discovered by Franz Joseph Muller von Reichstein in 1783 at Romania. Origin of name: from the Latin word "tellus" meaning "earth".Tellurium: isotopes
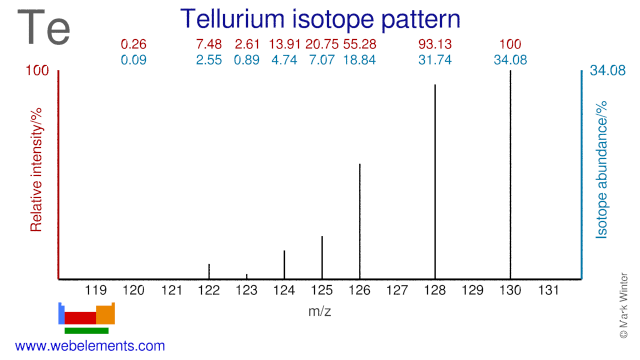
Tellurium has eight stable isotopes (Te-120 and Te-123 are usually considered stable because of their long half lives) and many of them have a medical application. Te-120 is used for the production of I-120g which has an application as a PET and Beta emitting isotope. Te-122 is used in the production of the radioisotope I-122 which is used in gamma imaging. Te-123 is used for the production of radioactive I-123 which is used in thyroid imaging. Te-124 is used for the production of both I-123 and the PET isotope I-124. Finally, Te-130 is used in the research into double Beta decay.
Tellurium: isolation
Isolation: it is not usually necessary to make tellurium in the laboratory as it is commercially available. While there are some tellurium ores, most tellurium is made as a byproduct of copper refining. Extraction is complex since the method emplyed will depend upon what other compounds or elements are present. The first step usually involves an oxidation in the presence of sodium carbonate (soda ash).
Cu2Te + Na2CO3 + 2O2 → 2CuO + Na2TeO3 + CO2
The tellurite Na2TeO3 is acidified with sulphuric acid and the tellurium precipitates out as the dioxide (leaving and selenous acid, H2SeO3, in solution). Tellurium is liberated from the dioxide by dissolving in sodium hydroxide, NaOH, and electroytic reduction.
TeO2 + 2NaOH → Na2TeO3 + H2O → Te + 2NaOH + O2