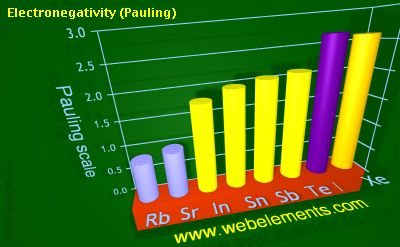
Electronegativity (Pauling)
"Electronegativity is the power of an atom when in a molecule to attract eletrons to itself." The electronegativity will depend upon a number of factors including other atoms in the molecule, the number of atoms coordinated to it, and the oxidation number for the atom. There are a number of ways to produce a set of numbers which represent electronegativity scales. The Pauling scale is perhaps the most famous.
He noticed that the bond energy E(AB) in a molecule AB is always greater than the mean of the bond energies E(AA) + E(BB) in the homonuclear species AA and BB. His argument was that in an "ideal" covalent bond E(AB) should equal this mean, and that the "excess" bond energy is caused by electrostatic attraction between the partially charged atoms in the heternuclear species AB. In effect, he was saying that the excess bond energy arises from an ionic contribution to the bond. He managed to treat this ionic contribution by the equation
E(AB) = [E(AA).E(BB)]1/2 + 96.48(ΧA - ΧB)2
in which E(AB) is expressed in kJ mol-1 (1 electron volt, 1eV, = 96.48 kJ mol-1) and ΧA - ΧB represents the difference in "electronegativity" between the two elements, whose individual electronegativities are given the symbols ΧA and Χ. Using this equation, Pauling found that the largest electronegativity difference was between Cs and F. Pauling set F arbitrarily at 4.0 (today, the value for F is set to 3.98) and this gives a scale in which the values for all other elements are less than 4 but still with a positive number.
Units
Pauling scale
Notes
Most values are taken from reference 1 and where values are missing from reference 2. Values for Group 18 elements and for elements 95-102 are taken from reference 3. Pauling electronegativities are published in many sources and essentially identical data are to be found in references such as 4 and 5.
You can look at visual representations of the various electronegativity scales using the following links.
- Electronegativity
- Electronegativity (Allen)
- Electronegativity (Allred-Rochow)
- Electronegativity (Pauling)
- Electronegativity (Mulliken-Jaffe)
- Electronegativity (Mulliken-Jaffe) p-orbital
- Electronegativity (Mulliken-Jaffe - s)
- Electronegativity (Mulliken-Jaffe - sp)
- Electronegativity (Mulliken-Jaffe -sp2)
- Electronegativity (Mulliken-Jaffe -sp3)
- Electronegativity (Sanderson)
Literature sources
- A.L. Allred, J. Inorg. Nucl. Chem., 1961, 17, 215.
- L. Pauling, The Nature of the Chemical Bond, Cornell Univ., USA, 3rd ed., 1960.
- J.E. Huheey, E.A. Keiter, and R.L. Keiter in Inorganic Chemistry : Principles of Structure and Reactivity, 4th edition, HarperCollins, New York, USA, 1993.
- W.W. Porterfield in Inorganic chemistry, a unified approach, Addison Wesley Publishing Co., Reading Massachusetts, USA, 1984.
- A.M. James and M.P. Lord in Macmillan's Chemical and Physical Data, Macmillan, London, UK, 1992.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1
|
2
|
|||||||||||||||||
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
10
|
|||||||||||
|
11
|
12
|
13
|
14
|
15
|
16
|
17
|
18
|
|||||||||||
|
19
|
20
|
21
|
22
|
23
|
24
|
25
|
26
|
27
|
28
|
29
|
30
|
31
|
32
|
33
|
34
|
35
|
36
|
|
|
37
|
38
|
39
|
40
|
41
|
42
|
43
|
44
|
45
|
46
|
47
|
48
|
49
|
50
|
51
|
52
|
53
|
54
|
|
|
55
|
56
|
* |
71
|
72
|
73
|
74
|
75
|
76
|
77
|
78
|
79
|
80
|
81
|
82
|
83
|
84
|
85
|
86
|
|
87
|
88
|
** |
103
|
104
|
105
|
106
|
107
|
108
|
109
|
110
|
111
|
112
|
113
|
114
|
115
|
116
|
117
|
118
|
| *Lanthanoids | * |
57
|
58
|
59
|
60
|
61
|
62
|
63
|
64
|
65
|
66
|
67
|
68
|
69
|
70
|
|||
| **Actinoids | ** |
89
|
90
|
91
|
92
|
93
|
94
|
95
|
96
|
97
|
98
|
99
|
100
|
101
|
102
|
|||