Electronegativity (Allred-Rochow)
Allred and Rochow suggest a scale of electronegativity based upon the electrostatic force of attraction between the nucleus and the valence electrons. The values expressed here are converted to the Pauling scale (see below). The Allred-Rochow electronegativity is often denoted as ΧAR
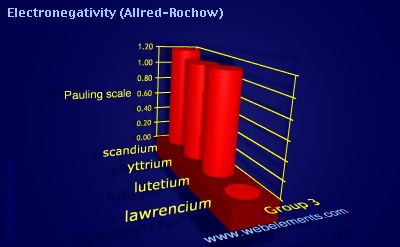
Units
Pauling scale
Notes
Most values are taken from reference 1. Additional values for elements 40-46 and 58 onwards are contained within reference 2. Values for elements 95-102 are estimates. Values for Group 18 elements are taken from reference 3. Essentially similar data for Allred-Rochow electronegativities are given in references 4 and 5, as well as many text books.
The force of attraction is given by:
force = e2Zeff/r2
where
- r is the distance between the electron and the nucleus (covalent radius)
- e is the charge on an electron
- eZeff is the charge effective at the electron due to the nucleus and its surrounding electrons
The quantity Zeff/r2 correlates well with Pauling electronegativities and the two scales can be made to coincide by expressing the Allred-Rochow electronegativity as:
ΧAR = 0.744 + 0.359Zeff/r2
You can look at visual representations of the various electronegativity scales using the following links.
- Electronegativity
- Electronegativity (Allen)
- Electronegativity (Allred-Rochow)
- Electronegativity (Pauling)
- Electronegativity (Mulliken-Jaffe)
- Electronegativity (Mulliken-Jaffe) p-orbital
- Electronegativity (Mulliken-Jaffe - s)
- Electronegativity (Mulliken-Jaffe - sp)
- Electronegativity (Mulliken-Jaffe -sp2)
- Electronegativity (Mulliken-Jaffe -sp3)
- Electronegativity (Sanderson)
Literature sources
- A.L. Allred and E.G. Rochow, J. Inorg. Nucl. Chem., 1958, 5, 264.
- E.J. Little and M.M. Jones, J. Chem. Ed., 1960, 37, 231.
- J.E. Huheey, E.A. Keiter, and R.L. Keiter in Inorganic Chemistry : Principles of Structure and Reactivity, 4th edition, HarperCollins, New York, USA, 1993.
- W.W. Porterfield in Inorganic chemistry, a unified approach, Addison Wesley Publishing Co., Reading Massachusetts, USA, 1984.
- A.M. James and M.P. Lord in Macmillan's Chemical and Physical Data, Macmillan, London, UK, 1992.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1
|
2
|
|||||||||||||||||
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
10
|
|||||||||||
|
11
|
12
|
13
|
14
|
15
|
16
|
17
|
18
|
|||||||||||
|
19
|
20
|
21
|
22
|
23
|
24
|
25
|
26
|
27
|
28
|
29
|
30
|
31
|
32
|
33
|
34
|
35
|
36
|
|
|
37
|
38
|
39
|
40
|
41
|
42
|
43
|
44
|
45
|
46
|
47
|
48
|
49
|
50
|
51
|
52
|
53
|
54
|
|
|
55
|
56
|
* |
71
|
72
|
73
|
74
|
75
|
76
|
77
|
78
|
79
|
80
|
81
|
82
|
83
|
84
|
85
|
86
|
|
87
|
88
|
** |
103
|
104
|
105
|
106
|
107
|
108
|
109
|
110
|
111
|
112
|
113
|
114
|
115
|
116
|
117
|
118
|
| *Lanthanoids | * |
57
|
58
|
59
|
60
|
61
|
62
|
63
|
64
|
65
|
66
|
67
|
68
|
69
|
70
|
|||
| **Actinoids | ** |
89
|
90
|
91
|
92
|
93
|
94
|
95
|
96
|
97
|
98
|
99
|
100
|
101
|
102
|
|||