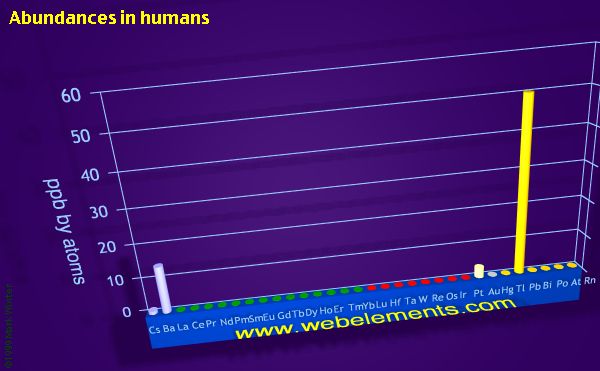
Abundances in humans (by atoms)
The values here are estimates adapted from references 1-6 by taking a consensus or averaging where appropriate. For ease of comparison with other abundance values, all abundance data in WebElements have been scaled to parts per billion (ppb), both as ppb by weight and by number of atoms. Values for less common elements should be treated with some caution and none of the data is better than one or sometimes two significant figures. Values for any individual human will depend very much upon that individual's local environment. For instance, if you live near a major road in a country where leaded fuel is still sold, you are likely to have more lead in you than the average.
Units
ppb by atoms
Notes
The units used in WebElements for all abundance data are ppb by weight which means parts per billion by weight, that is mg tonne-3 or mg per 1000 kg. All abundance data are also presented as ppb by atoms, which means atoms of the element per billion atoms.
The reason for rescaling all data is as follows. It is common to see, say, solar abundances expressed as the number of atoms of the element relative to a scale upon which the abundance of hydrogen is defined as 1012. This makes comparison with, say, crustal abundances difficult, since crustal abundances are often expressed in terms of parts per million by weight. Hence a common scale is used throughout and I chose ppb as this gives manageable numbers for most elements.
For access to other abundance data as ppb by weight, select from:
For access to other abundance data as ppb by atoms, select from:
Literature sources
- I.S. Butler and J.F. Harrod in Inorganic Chemistry, Principles and Applications, Benjamin Cummings, California, USA, 1989.
- H.J.M. Bowen in Environmental Chemistry of the elements, Academic Press, London, UK, 1979.
- A.M. James and M.P. Lord in Macmillan's Chemical and Physical Data, Macmillan, London, UK, 1992.
- P.A. Cox in The Elements : Their Origin, Abundance, and Distribution, Oxford University Press, Oxford, UK, 1989.
- D.R. Lide, (Ed.) in Chemical Rubber Company handbook of chemistry and physics, CRC Press, Boca Raton, Florida, USA, 79th edition, 1998.
- J. Selbin, J. Chem. Ed. 1973, 50, 306.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1
|
2
|
|||||||||||||||||
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
10
|
|||||||||||
|
11
|
12
|
13
|
14
|
15
|
16
|
17
|
18
|
|||||||||||
|
19
|
20
|
21
|
22
|
23
|
24
|
25
|
26
|
27
|
28
|
29
|
30
|
31
|
32
|
33
|
34
|
35
|
36
|
|
|
37
|
38
|
39
|
40
|
41
|
42
|
43
|
44
|
45
|
46
|
47
|
48
|
49
|
50
|
51
|
52
|
53
|
54
|
|
|
55
|
56
|
* |
71
|
72
|
73
|
74
|
75
|
76
|
77
|
78
|
79
|
80
|
81
|
82
|
83
|
84
|
85
|
86
|
|
87
|
88
|
** |
103
|
104
|
105
|
106
|
107
|
108
|
109
|
110
|
111
|
112
|
113
|
114
|
115
|
116
|
117
|
118
|
| *Lanthanoids | * |
57
|
58
|
59
|
60
|
61
|
62
|
63
|
64
|
65
|
66
|
67
|
68
|
69
|
70
|
|||
| **Actinoids | ** |
89
|
90
|
91
|
92
|
93
|
94
|
95
|
96
|
97
|
98
|
99
|
100
|
101
|
102
|
|||