Nitrogen - 7N: the essentials
- Name: nitrogen
- Symbol: N
- Atomic number: 7
- Relative atomic mass (Ar): 14.007 range: [14.00643, 14.00728] [see notes g r]
- Standard state: gas at 298 K
- Appearance: colourless
- Classification: Non-metallic
- Group in periodic table: 15
- Group name: Pnictogen
- Period in periodic table: 2
- Block in periodic table: p
- Shell structure: 2.5
- CAS Registry: 7727-37-9
Nitrogen atoms have 7 electrons and the shell structure is 2.5. The ground state electronic configuration of neutral nitrogen is [He].2s2.2p3 and the term symbol of nitrogen is 4S3/2.
Nitrogen: description
Nitrogen is a Group 15 element. Nitrogen makes up about 78% of the atmosphere by volume but the atmosphere of Mars contains less than 3% nitrogen. The element seemed so inert that Lavoisier named it azote, meaning "without life". However, its compounds are vital components of foods, fertilizers, and explosives. Nitrogen gas is colourless, odourless, and generally inert. As a liquid it is also colourless and odourless.
When nitrogen is heated, it combines directly with magnesium, lithium, or calcium. When mixed with oxygen and subjected to electric sparks, it forms nitric oxide (NO) and then the dioxide (NO2). When heated under pressure with hydrogen in the presence of a suitable catalyst , ammonia forms (Haber process). Nitrogen is "fixed" from the atmosphere by bacteria in the roots of certain plants such as clover. Hence the usefulness of clover in crop rotation.

Image adapted with permission from Prof James Marshall's (U. North Texas, USA) Walking Tour of the elements CD.
Nitrogen: physical properties
Density of solid: 1026 kg m-3
Molar volume: 13.54 cm3
Thermal conductivity: 0.02583 W m‑1 K‑1
Nitrogen: heat properties
Melting point: 63.05 [‑210.1 °C (‑346.18 °F)] K
Boiling point: 77.36 [‑195.79 °C (‑320.42 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Nitrogen: atom sizes
Atomic radius (empirical): 65 pm
Molecular single bond covalent radius: 71 (coordination number 3) ppm
van der Waals radius: 166 ppm
Nitrogen: electronegativities
Pauling electronegativity: 3.04 (Pauling units)
Allred Rochow electronegativity: 3.07 (Pauling units)
Mulliken-Jaffe electronegativity: 2.90 (20% s orbital)
Nitrogen: orbital properties
First ionisation energy: 1402.33 kJ mol‑1
Second ionisation energy: 2856.09 kJ mol‑1
Third ionisation energy: 4577.77 kJ mol‑1
Nitrogen: abundances
Universe: 1000000 ppb by weight
Crustal rocks: 20000 ppb by weight
Human: 26000000 ppb by weight
Nitrogen: crystal structure
Nitrogen: biological data
Human abundance by weight: 26000000 ppb by weight
Nitrogen is a key component of biological molecules such as proteins (which are made from amino acids, and nucleic acids. The nitrogen cycle in nature is very important.
Nitrogen: uses
Nitrogen: reactions
Reactions of nitrogen as the element with air, water, halogens, acids, and bases where known.
Nitrogen: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of nitrogen where known.
Nitrogen: compound properties
Bond strengths; lattice energies of nitrogen halides, hydrides, oxides (where known); and reduction potentials where known.
Nitrogen: history
Nitrogen was discovered by Daniel Rutherford in 1772 at Scotland. Origin of name: from the Greek words "nitron genes" meaning "nitre" and "forming" and the Latin word "nitrum" (nitre is a common name for potassium nitrate, KNO#).Nitrogen: isotopes
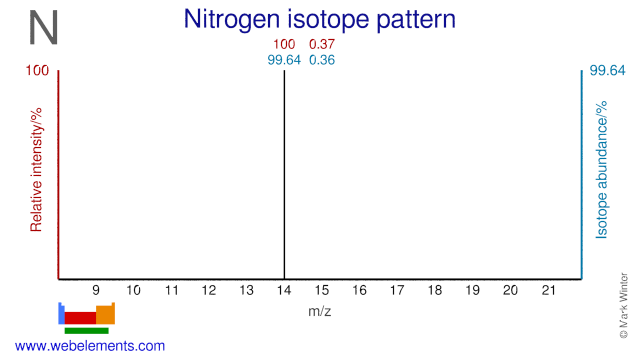
Nitrogen has two isotopes, N-14 and N-15, both of which are used in various applications. N-15 is used for the production of the radioisotope O-15 which is used in PET. N-15 is also used to study the uptake of Nitrogen in plants and the metabolism of proteins in the human body. N-14 is used for the production of the PET radioisotope C-11. It can also be used for the production of the PET radioisotopes N-13 and O-15.
Nitrogen: isolation
Isolation: there is never any need to make nitrogen in the laboratory as it is readily available commercially or through in-house air liquefaction plants. However the decomposition of sodium azide is one route to N2 and decomposition is ammonium dichromate is another. Both reactions must only be carried out under controlled conditions by a professional.
NaN3 (300°C) → 2Na + 3N2
(NH4)2Cr2O7 → N2 + Cr2O3 + 4H2O
Nitrogen is made on massive scale by liquefaction of air and fractional distillation of the resulting liquid air to separate out oxygen and other gases. Very high purity nitrogen is available by this route.