Hafnium - 72Hf: the essentials
- Name: hafnium
- Symbol: Hf
- Atomic number: 72
- Relative atomic mass (Ar): 178.49 (2)
- Standard state: solid at 298 K
- Appearance: grey steel
- Classification: Metallic
- Group in periodic table: 4
- Group name: (none)
- Period in periodic table: 6
- Block in periodic table: d
- Shell structure: 2.8.18.32.10.2
- CAS Registry: 7440-58-6
Hafnium atoms have 72 electrons and the shell structure is 2.8.18.32.10.2. The ground state electronic configuration of neutral hafnium is [Xe].4f14.5d2.6s2 and the term symbol of hafnium is 3F2.
Hafnium: description
Most zirconium minerals contain 1 to 3% hafnium. Hafnium is a ductile metal with a brilliant silver lustre. Its properties are influenced considerably by the impurities of zirconium present. Of all the elements, zirconium and hafnium are two of the most difficult to separate. Hafnium is a Group 4 transition element.
Because hafnium has a good absorption cross section for thermal neutrons (almost 600 times that of zirconium), has excellent mechanical properties, and is extremely corrosion resistant, it is used for nuclear reactor control rods.
Hafnium carbide is the most refractory binary composition known, and the nitride is the most refractory metal nitride (m.p. 3310°C).

Hafnium foil.
Hafnium: physical properties
Density of solid: 13310 kg m-3
Molar volume: 13.44 cm3
Thermal conductivity: 23 W m‑1 K‑1
Hafnium: heat properties
Melting point: 2506 [2233 °C (4051 °F)] K
Boiling point: 4876 [4603 °C (8317 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Hafnium: atom sizes
Atomic radius (empirical): 155 pm
Molecular single bond covalent radius: 152 (coordination number 4) ppm
van der Waals radius: 253 ppm
Hafnium: electronegativities
Pauling electronegativity: 1.3 (Pauling units)
Allred Rochow electronegativity: 1.23 (Pauling units)
Mulliken-Jaffe electronegativity: (no data)
Hafnium: orbital properties
First ionisation energy: 658.52 kJ mol‑1
Second ionisation energy: 1410 kJ mol‑1
Third ionisation energy: 2176 kJ mol‑1
Hafnium: abundances
Universe: 0.7 ppb by weight
Crustal rocks: 3300 ppb by weight
Human: (no data) ppb by weight
Hafnium: crystal structure
Hafnium: biological data
Human abundance by weight: (no data) ppb by weight
Hafnium has no biological role.
Hafnium: uses
Hafnium: reactions
Reactions of hafnium as the element with air, water, halogens, acids, and bases where known.
Hafnium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of hafnium where known.
Hafnium: compound properties
Bond strengths; lattice energies of hafnium halides, hydrides, oxides (where known); and reduction potentials where known.
Hafnium: history
Hafnium was discovered by Dirk Coster and George Charles von Hevesy in 1923 at Denmark. Origin of name: from the Latin name "Hafnia" meaning "Copenhagen".Hafnium: isotopes
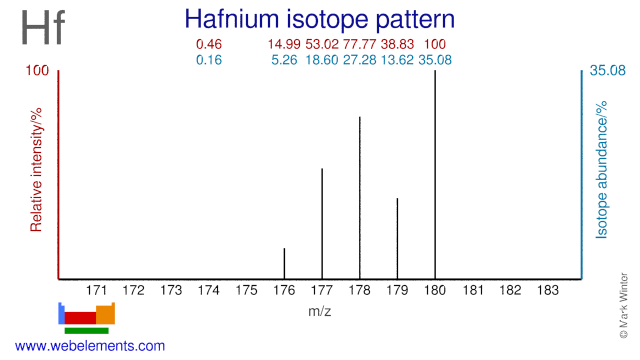
Hafnium isotopes have several applications. Hf-180 is used for the production of the radioisotope Hf-181 while Hf-180 is used for the production of the radioisotope Ta-179, which has a medical application. The second isomer of Hf-178 (Hf-178m2) exhibits a very high excitation energy and it has been suggested for use in gamma ray lasers.
Hafnium: isolation
Isolation: hafnium extraction is always associated with its removal from zirconium as it is a contaminant of all zirconium minerals. Solvent extraction methods are used ot spearate the two metals but the process is not easy. These make use of the differential solubilities of the metal thiocyantes (thiocyanate is SCN-) in methyl isobutyl ketone.