Calcium - 20Ca: the essentials
- Name: calcium
- Symbol: Ca
- Atomic number: 20
- Relative atomic mass (Ar): 40.078 (4) g [see note g]
- Standard state: solid at 298 K
- Appearance: silvery white
- Classification: Metallic
- Group in periodic table: 2
- Group name: Alkaline earth metal
- Period in periodic table: 4
- Block in periodic table: s
- Shell structure: 2.8.8.2
- CAS Registry: 7440-70-2
Calcium atoms have 20 electrons and the shell structure is 2.8.8.2. The ground state electronic configuration of neutral calcium is [Ar].4s2 and the term symbol of calcium is 1S0.
Calcium: description
Calcium as the element is a grey silvery metal. The metal is rather hard. Calcium is an essential constituent of leaves, bones, teeth, and shells. Calcium is the fifth most abundant element in the earth's crust and makes up more than 3% of the crust. Calcium does not occur as the metal itself in nature and instead is found in various minerals including as limestone, gypsum and fluorite. Stalagmites and stalactites contain calcium carbonate (CaCO3). Calcium carbonate is the basis of the cement industry.
Calcium is classified chemically as one of the alkaline earth elements (that is, in Group 2 of the periodic table. The metal is rather reactive. It readily forms a white coating of calcium nitride (Ca3N2) in air. It reacts with water and the metal burns with a yellow-red flame, forming largely the nitride.

This sample is from The Elements Collection, an attractive and safely packaged collection of the 92 naturally occurring elements that is available for sale.
Calcium: physical properties
Density of solid: 1550 kg m-3
Molar volume: 26.20 cm3
Thermal conductivity: 200 W m‑1 K‑1
Calcium: heat properties
Melting point: 1115 [842 °C (1548 °F)] K
Boiling point: 1757 [1484 °C (2703 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Calcium: atom sizes
Atomic radius (empirical): 180 pm
Molecular single bond covalent radius: 171 (coordination number 2) ppm
van der Waals radius: 262 ppm
Calcium: electronegativities
Pauling electronegativity: 1.00 (Pauling units)
Allred Rochow electronegativity: 1.04 (Pauling units)
Mulliken-Jaffe electronegativity: 1.08 (sp orbital)
Calcium: orbital properties
First ionisation energy: 589.83 kJ mol‑1
Second ionisation energy: 1145.45 kJ mol‑1
Third ionisation energy: 4912.37 kJ mol‑1
Calcium: abundances
Universe: 70000 ppb by weight
Crustal rocks: 50000000 ppb by weight
Human: 14000000 ppb by weight
Calcium: crystal structure
Calcium: biological data
Human abundance by weight: 14000000 ppb by weight
Calcium is essential for all life. It forms part of cell walls and bones. It is important for blood clotting.
Calcium: uses
Calcium: reactions
Reactions of calcium as the element with air, water, halogens, acids, and bases where known.
Calcium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of calcium where known.
Calcium: compound properties
Bond strengths; lattice energies of calcium halides, hydrides, oxides (where known); and reduction potentials where known.
Calcium: history
Calcium was discovered by Sir Humphrey Davy in 1808 at England. Origin of name: from the Latin word "calx" meaning "lime".Calcium: isotopes
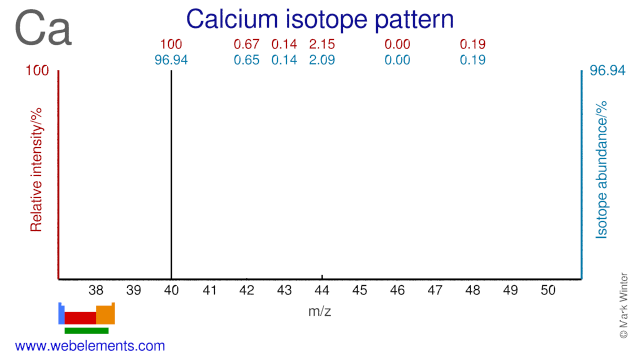
Calcium isotopes (mainly Ca-42, Ca-44, Ca-46 and Ca-48) are used extensively in clinical research and mainly in nutritional studies. They are used to measure calcium absorption mainly in women and children. In adults, calcium deficiency is strongly related to increasing severity of osteoporosis. In children, calcium deficiency is primarily related to the development of rickets. Ca-48 has been used to bombard Pb and Bi targets in order to create super heavy elements.
Calcium: isolation
Isolation: calcium metal is readily available commercially and there is no need to make it in the laboratory. Commercially it can be made by the electrolysis of molten calcium chloride, CaCl2.
cathode: Ca2+(l) + 2e- → Ca
anode: Cl-(l) → 1/2Cl2 (g) + e-
The calcium chloride is made by the action of hydrochloric acid upon calcium carbonate. Calcium chloride is also a byproduct in the Solway process used to make sodium carbonate.
CaCO3 + 2HCl → CaCl2 + H2O + CO2
Alternatively, and on small scale, calcium can be made through the reduction of CaO with aluminium or of CaCl2 with sodium metal
6CaO + 2Al→ 3Ca + Ca3Al2O6
CaCl2 + 2Na→ Ca + 2NaCl