Caesium - 55Cs: the essentials
- Name: caesium
- Symbol: Cs
- Atomic number: 55
- Relative atomic mass (Ar): 132.90545196 (6)
- Standard state: solid at 298 K (but melts only slightly above this temperature)
- Appearance: silvery gold
- Classification: Metallic
- Group in periodic table: 1
- Group name: Alkali metal
- Period in periodic table: 6
- Block in periodic table: s
- Shell structure: 2.8.18.18.8.1
- CAS Registry: 7440-46-2
Caesium atoms have 55 electrons and the shell structure is 2.8.18.18.8.1. The ground state electronic configuration of neutral caesium is [Xe].6s1 and the term symbol of caesium is 2S1/2.
Caesium: description
Caesium is known as cesium in the USA.
The metal is characterised by a spectrum containing two bright lines in the blue (accounting for its name). It is silvery gold, soft, and ductile. It is the most electropositive and most alkaline element. Caesium, gallium, and mercury are the only three metals that are liquid at or around room temperature. Caesium reacts explosively with cold water, and reacts with ice at temperatures above -116°C. Caesium hydroxide is a strong base and attacks glass.
Cartoon by Nick D Kim ([Science and Ink], used by permission).

Image adapted with permission from Prof James Marshall's (U. North Texas, USA) Walking Tour of the elements CD.
Caesium: physical properties
Density of solid: 1879 kg m-3
Molar volume: 70.94 cm3
Thermal conductivity: 36 W m‑1 K‑1
Caesium: heat properties
Melting point: 301.59 [28.44 °C (83.19 °F)] K
Boiling point: 944 [671 °C (1240 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Caesium: atom sizes
Atomic radius (empirical): 260 pm
Molecular single bond covalent radius: 232 (coordination number 1) ppm
van der Waals radius: 348 ppm
Caesium: electronegativities
Pauling electronegativity: 0.79 (Pauling units)
Allred Rochow electronegativity: 0.86 (Pauling units)
Mulliken-Jaffe electronegativity: 0.62 (s orbital)
Caesium: orbital properties
First ionisation energy: 375.70 kJ mol‑1
Second ionisation energy: 2234.35 kJ mol‑1
Third ionisation energy: 3202.8 kJ mol‑1
Caesium: abundances
Universe: 0.8 ppb by weight
Crustal rocks: 1900 ppb by weight
Human: 20 ppb by weight
Caesium: crystal structure

Caesium: biological data
Human abundance by weight: 20 ppb by weight
Caesium (cesium in USA) has no biological role. However it is capable of replacing potassium in the body to some extent because of its chemical similarity. Ingestion of any caesium compounds is therefore to be avoided. Because of this similarity, the isotopes 134Cs and 137Cs (present in the biosphere in small amounts as a result of radiation leaks) are very toxic. Rats fed caesium in place of potassium in their diet die.
Caesium: uses
Caesium: reactions
Reactions of caesium as the element with air, water, halogens, acids, and bases where known.
Caesium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of caesium where known.
Caesium: compound properties
Bond strengths; lattice energies of caesium halides, hydrides, oxides (where known); and reduction potentials where known.
Caesium: history
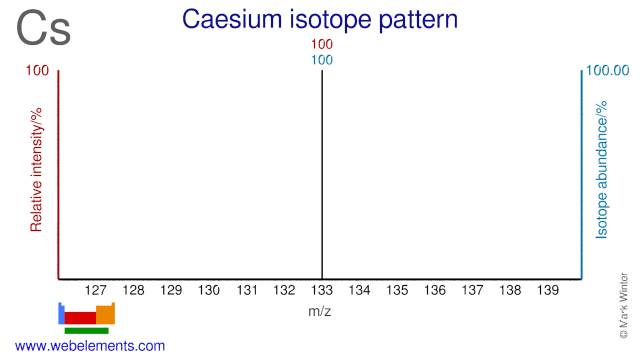
Caesium was discovered by Gustav Kirchhoff, Robert Bunsen in 1860 at Germany. Origin of name: from the Latin word "caesius" meaning "sky blue" or "heavenly blue".Caesium: isotopes
Caesium: isolation
Isolation: caesium (cesium in USA) would not normally be made in the laboratory as it is available commercially. All syntheses require an electrolytic step as it is so difficult to add an electron to the poorly electronegative caesium ion Cs+.
Caesium is not made by the same method as sodium as might have been expected. This is because the caesium metal, once formed by electrolysis of liquid caesium chloride (CsCl), is too soluble in the molten salt.
cathode: Cs+(l) + e- → Cs (l)
anode: Cl-(l) → 1/2Cl2 (g) + e-
Instead, it is made by the reaction of metallic sodium with hot molten caesium chloride.
Na + CsCl ⇌ Cs + NaCl
This is an equilbrium reaction and under these conditions the caesium is highly volatile and removed from the system in a form relatively free from sodium impurities, allowing the reaction to proceed. It can be purified by distillation.
