Beryllium - 4Be: the essentials
- Name: beryllium
- Symbol: Be
- Atomic number: 4
- Relative atomic mass (Ar): 9.0121831 (5)
- Standard state: solid at 298 K
- Appearance: lead grey
- Classification: Metallic
- Group in periodic table: 2
- Group name: Alkaline earth metal
- Period in periodic table: 2
- Block in periodic table: s
- Shell structure: 2.2
- CAS Registry: 7440-41-7
Beryllium atoms have 4 electrons and the shell structure is 2.2. The ground state electronic configuration of neutral beryllium is [He].2s2 and the term symbol of beryllium is 1S0.
Beryllium: description
Beryllium is a Group 2 (IIA) element. It is a metal and has a high melting point. At ordinary temperatures, beryllium resists oxidation in air. Beryllium compounds are very toxic. Its ability to scratch glass is probably due to the formation of a thin layer of the oxide. Aquamarine and emerald are precious forms of the mineral beryl, [Be3Al2(SiO3)6].
Its chemistry is dominated by its tendency to lose an electron to form Be2+. As this ion is so small it is highly polarising, to the extent that its compounds are rather covalent. Its small size means that its complexes tend to be tetrahedral rahter than octahedral.
Beryllium: physical properties
Density of solid: 1848 kg m-3
Molar volume: 4.85 cm3
Thermal conductivity: 190 W m‑1 K‑1
Beryllium: heat properties
Melting point: 1560 [1287 °C (2349 °F)] K
Boiling point: 2742 [2469 °C (4476 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Beryllium: atom sizes
Atomic radius (empirical): 105 pm
Molecular single bond covalent radius: 102 (coordination number 2) ppm
van der Waals radius: 198 ppm
Beryllium: electronegativities
Pauling electronegativity: 1.57 (Pauling units)
Allred Rochow electronegativity: 1.47 (Pauling units)
Mulliken-Jaffe electronegativity: 1.54 (s orbital)
Beryllium: orbital properties
First ionisation energy: 899.50 kJ mol‑1
Second ionisation energy: 1757.11 kJ mol‑1
Third ionisation energy: 14848.72 kJ mol‑1
Beryllium: abundances
Universe: 1 ppb by weight
Crustal rocks: 1900 ppb by weight
Human: 0.4 ppb by weight
Beryllium: crystal structure
Beryllium: biological data
Human abundance by weight: 0.4 ppb by weight
Beryllium has no biological role. In fact, compounds containing beryllium are poisonous.
Beryllium: uses
Beryllium: reactions
Reactions of beryllium as the element with air, water, halogens, acids, and bases where known.
Beryllium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of beryllium where known.
Beryllium: compound properties
Bond strengths; lattice energies of beryllium halides, hydrides, oxides (where known); and reduction potentials where known.
Beryllium: history
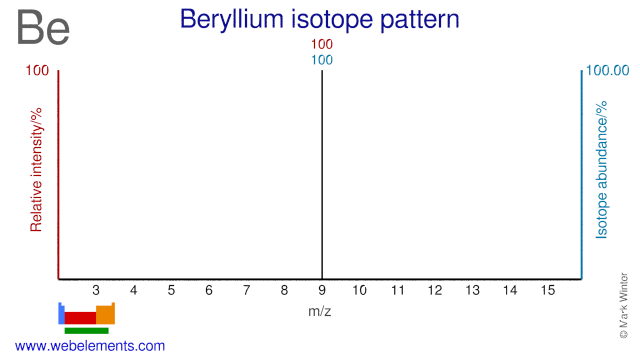
Beryllium was discovered by Nicholas Louis Vauquelin (1763-1829) in 1797 at France. Origin of name: from the Greek word "beryllos" meaning "beryl".Beryllium: isotopes
Beryllium: isolation
Isolation: beryllium metal is available commercially and so would never normally be made in the laboratory. Its extraction from ores is complex. The mineral beryl, [Be3Al2(SiO3)6] is the most important source of beryllium. It is roasted with sodimu hexafluorosilicate, Na2SiF6, at 700°C to form beryllium fluoride. This is water soluble and the beryllium may be precipitated as the hydroxide Be(OH)2 by adjustment of the pH to 12.
Pure beryllium may be obtained by electrolysis of molten BeCl2 containing some NaCl. The salt is added since the molten BeCl2 conducts very poorly. Another method involves the reduction of beryllium fluoride with magnesium at 1300°C.
BeF2 + Mg → MgF2 + Be