Uranium - 92U: the essentials
- Name: uranium
- Symbol: U
- Atomic number: 92
- Relative atomic mass (Ar): 238.02891 (3) [see notes g m
- Standard state: solid at 298 K
- Appearance: metallic grey
- Classification: Metallic
- Group in periodic table:
- Group name: Actinoid
- Period in periodic table: 7 (actinoid)
- Block in periodic table: f
- Shell structure: 2.8.18.32.21.9.2
- CAS Registry: 7440-61-1
Uranium atoms have 92 electrons and the shell structure is 2.8.18.32.21.9.2. The ground state electronic configuration of neutral uranium is [Rn].5f3.6d1.7s2 and the term symbol of uranium is 5L6.
Uranium: description
Uranium is of great interest because of its application to nuclear power and nuclear weapons. Uranium contamination is an emotive environmental problem. It is not particularly rare and is more common than beryllium or tungsten for instance.

This sample is from The Elements Collection, an attractive and safely packaged collection of the 92 naturally occurring elements that is available for sale.

Cartoon by Nick D Kim ([Science and Ink], used by permission).
Uranium gives interesting yellow and green colours and fluorescence effects when included to glass in conjunction with other additives. The image below is an English amphora dating to about 1930 showing a characteristic yellow-green colour. The image is reproduced with the permission of Ken Tomabechi (Uranium Glass Gallery in Japan), where you can find further information about uranium glass. This type of glass is sometimes referred to as "vaseline glass" in the UK and USA and as "Annagelb" (yellow) or "Annagruen" (green) in Germany.

Uranium: physical properties
Density of solid: 19050 kg m-3
Molar volume: 12.49 cm3
Thermal conductivity: 27.6 W m‑1 K‑1
Uranium: heat properties
Melting point: 1405.3 [1132.2 °C (2070 °F)] K
Boiling point: 4200 [ca.3900 °C (7101 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Uranium: atom sizes
Atomic radius (empirical): 175 pm
Molecular single bond covalent radius: 170 (coordination number 3,6) ppm
van der Waals radius: [ 305 ] ppm
Uranium: electronegativities
Pauling electronegativity: 1.38 (Pauling units)
Allred Rochow electronegativity: 1.22 (Pauling units)
Mulliken-Jaffe electronegativity: (no data)
Uranium: orbital properties
First ionisation energy: 597.63 kJ mol‑1
Second ionisation energy: 1120 kJ mol‑1
Third ionisation energy: 1910 kJ mol‑1
Uranium: abundances
Universe: 0.2 ppb by weight
Crustal rocks: 1800 ppb by weight
Human: 1 ppb by weight
Uranium: crystal structure
Uranium: uses
Uranium: reactions
Reactions of uranium as the element with air, water, halogens, acids, and bases where known.
Uranium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of uranium where known.
Uranium: compound properties
Bond strengths; lattice energies of uranium halides, hydrides, oxides (where known); and reduction potentials where known.
Uranium: history
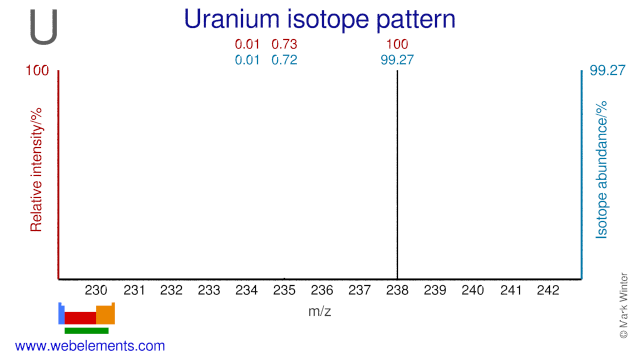
Uranium was discovered by Martin Klaproth in 1789 at Germany. Origin of name: named after "the planet Uranus".Uranium: isotopes
Uranium: isolation
Isolation: coming soon!