Bond enthalpy of diatomic M-Pb molecules
A definition will apppear here.
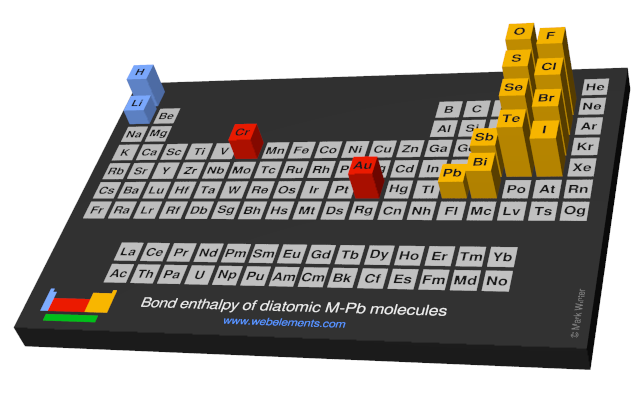
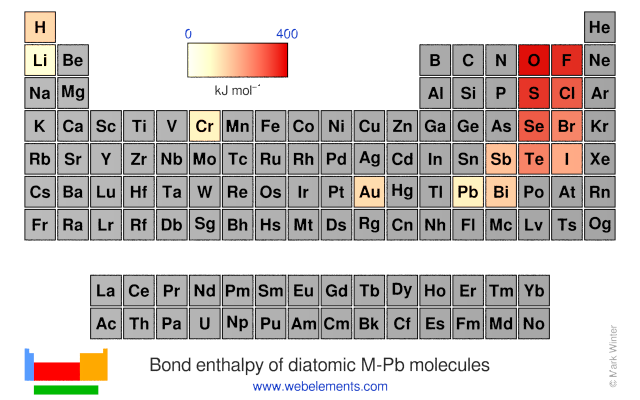
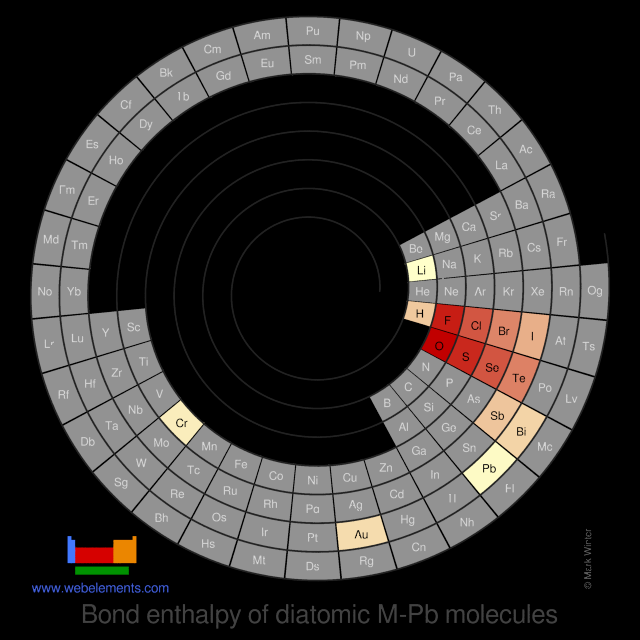
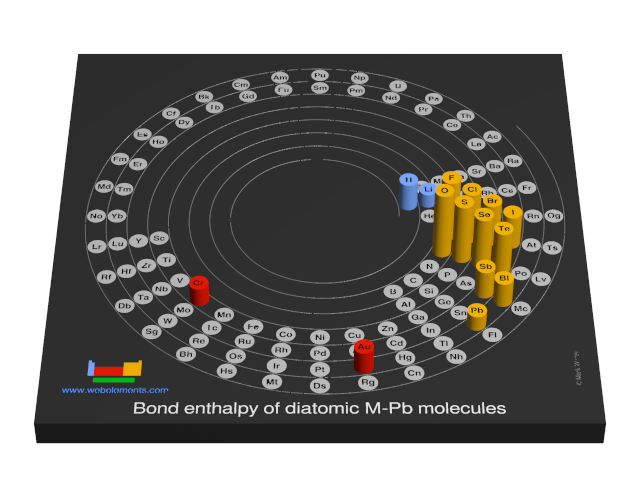
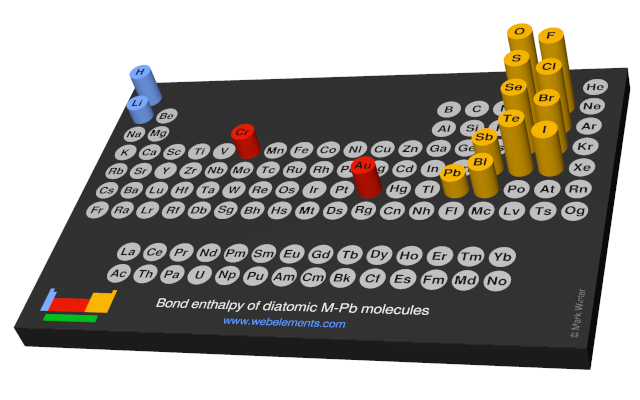
Units
kJ mol-1
Notes
I am grateful to Professor J.A. Kerr (University of Birmingham, UK) for the provision of the bond strengths of diatomic molecules data.
The values given here are at 298 K. All values are quoted in kJ mol-1. Generally, these data were obtained by spectroscopic or mass spectrometric means. You should consult reference 1 for further details. A note of caution: the strength of, say, the C-H bond in the gaseous diatomic species CH (not an isolatable species) is not necessarily, the same as the strength of a C-H bond in, say, methane.
Select from the following links to see visual periodicity representations for single element-halide single bond enthalpies in the highest halides or the single bond enthalpies for homodiatomic molecules M2.
- Single bond enthalpies in highest bromide
- Single bond enthalpies in highest chloride
- Single bond enthalpies in highest fluoride
- Single bond enthalpies in highest iodide
- Bond enthalpies (M-M single bond)
- Bond enthalpies for homodiatomic molecules M2 (not necessarily single bonds.
Each formula in the table below (M-O, M-F, and so on) is a link - select these to see visual periodicity representations for bond enthalpies involving your element of choice.
| Group 1 | Group 2 | Group 13 | Group 14 | Group 15 | Group 16 | Group 17 | Group 18 |
| M-H | M-He | ||||||
| M-Li | M-Be | M-B | M-C | M-N | M-O | M-F | M-Ne |
| M-Na | M-Mg | M-Al | M-Si | M-P | M-S | M-Cl | M-Ar |
| M-K | M-Ca | M-Ga | M-Ge | M-As | M-Se | M-Br | M-Kr |
| M-Rb | M-Sr | M-In | M-Sn | M-Sb | M-Te | M-I | M-Xe |
| M-Cs | M-Ba | M-Tl | M-Pb | M-Bi | M-Po (none) | M-At (none) | M-Rn |
| M-Fr | M-Ra | M-Nh (none) | M-Fl (none) | M-Mc (none) | M-Lv (none) | M-Ts (none) | M-Og (none) |
Literature sources
References to be added| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1
|
2
|
|||||||||||||||||
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
10
|
|||||||||||
|
11
|
12
|
13
|
14
|
15
|
16
|
17
|
18
|
|||||||||||
|
19
|
20
|
21
|
22
|
23
|
24
|
25
|
26
|
27
|
28
|
29
|
30
|
31
|
32
|
33
|
34
|
35
|
36
|
|
|
37
|
38
|
39
|
40
|
41
|
42
|
43
|
44
|
45
|
46
|
47
|
48
|
49
|
50
|
51
|
52
|
53
|
54
|
|
|
55
|
56
|
* |
71
|
72
|
73
|
74
|
75
|
76
|
77
|
78
|
79
|
80
|
81
|
82
|
83
|
84
|
85
|
86
|
|
87
|
88
|
** |
103
|
104
|
105
|
106
|
107
|
108
|
109
|
110
|
111
|
112
|
113
|
114
|
115
|
116
|
117
|
118
|
| *Lanthanoids | * |
57
|
58
|
59
|
60
|
61
|
62
|
63
|
64
|
65
|
66
|
67
|
68
|
69
|
70
|
|||
| **Actinoids | ** |
89
|
90
|
91
|
92
|
93
|
94
|
95
|
96
|
97
|
98
|
99
|
100
|
101
|
102
|
|||