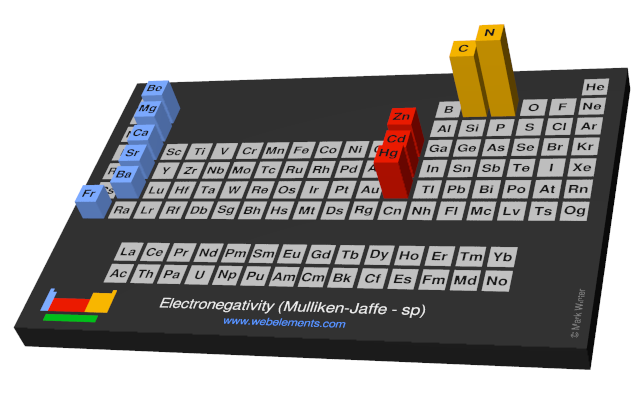
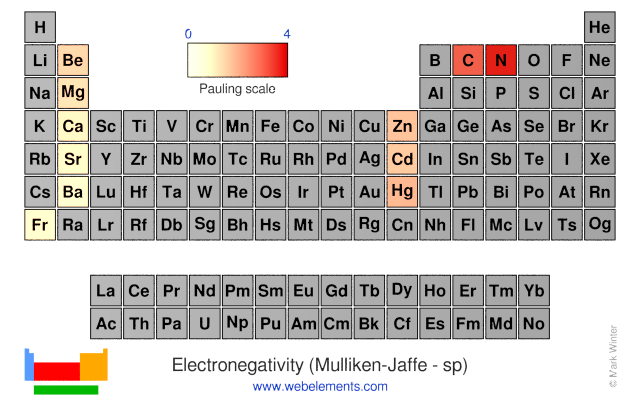
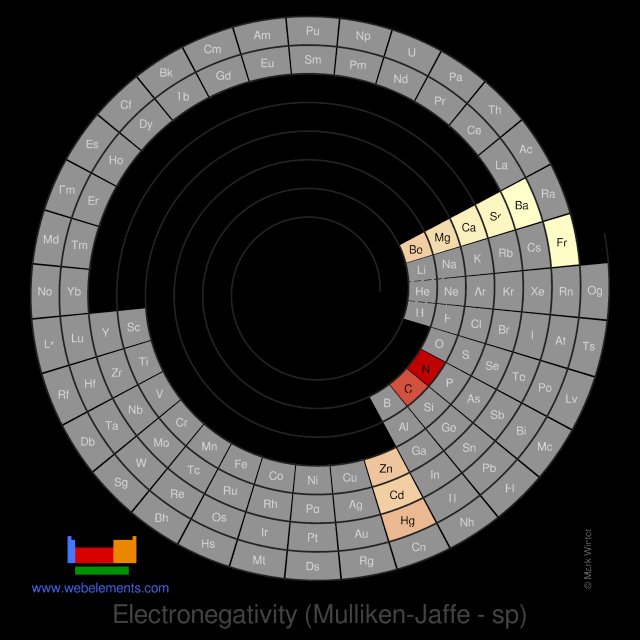
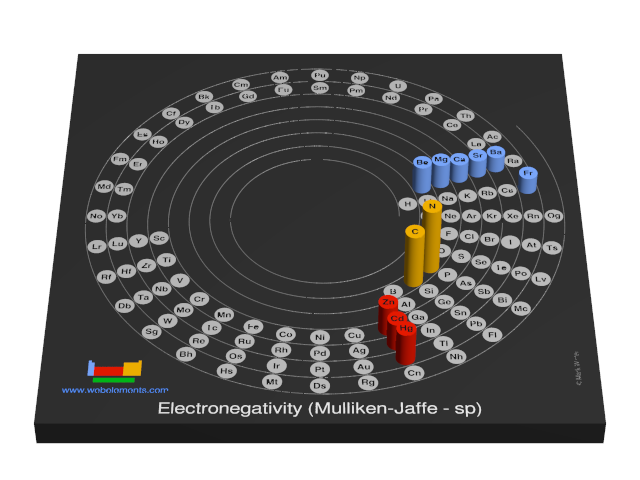
Electronegativity (Mulliken-Jaffe - sp)
R.S. Mulliken proposed an electronegativity scale in which the Mulliken electronegativity, ΧM is related to the electron affinity EAv (a measure of the tendency of an atom to form a negative species) and the ionization potential IEv (a measure of the tendency of an atom to form a positive species) by the equation:
ΧM = (IEv + EAv)/2
The subscript v denotes a specific valence state - so for trigonal boron compounds, a values of electronegativity can be defined for sp2 hybrid orbitals. If the values of IE and EA are in units of MJ mol-1, then the Mulliken electronegativity ΧM can be expressed on the Pauling scale by the relationship:
ΧM = 3.48[(IEv + EAv)/2 - 0.602]
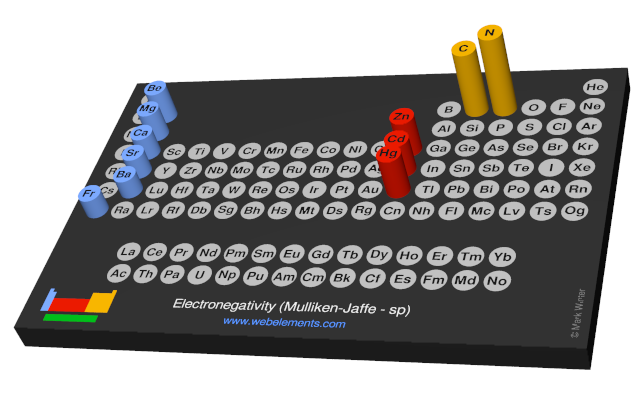
Units
Pauling scale
Notes
Values presented here are largely from reference 1. Mulliken's proposals are to be found in references 2 and 3. Tables of values are also given in references 4 and 5 as well.
You can look at visual representations of the various electronegativity scales using the following links.
- Electronegativity
- Electronegativity (Allen)
- Electronegativity (Allred-Rochow)
- Electronegativity (Pauling)
- Electronegativity (Mulliken-Jaffe)
- Electronegativity (Mulliken-Jaffe) p-orbital
- Electronegativity (Mulliken-Jaffe - s)
- Electronegativity (Mulliken-Jaffe - sp)
- Electronegativity (Mulliken-Jaffe -sp2)
- Electronegativity (Mulliken-Jaffe -sp3)
- Electronegativity (Sanderson)
Literature sources
- S.G. Bratsch, J. Chem. Ed., 1988, 65, 34.
- R.S. Mulliken, J. Chem. Phys., 1934, 2, 782.
- R.S. Mulliken, J. Chem. Phys., 1935, 3, 573.
- J.E. Huheey, E.A. Keiter, and R.L. Keiter in Inorganic Chemistry : Principles of Structure and Reactivity, 4th edition, HarperCollins, New York, USA, 1993.
- A.M. James and M.P. Lord in Macmillan's Chemical and Physical Data, Macmillan, London, UK, 1992.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1
|
2
|
|||||||||||||||||
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
10
|
|||||||||||
|
11
|
12
|
13
|
14
|
15
|
16
|
17
|
18
|
|||||||||||
|
19
|
20
|
21
|
22
|
23
|
24
|
25
|
26
|
27
|
28
|
29
|
30
|
31
|
32
|
33
|
34
|
35
|
36
|
|
|
37
|
38
|
39
|
40
|
41
|
42
|
43
|
44
|
45
|
46
|
47
|
48
|
49
|
50
|
51
|
52
|
53
|
54
|
|
|
55
|
56
|
* |
71
|
72
|
73
|
74
|
75
|
76
|
77
|
78
|
79
|
80
|
81
|
82
|
83
|
84
|
85
|
86
|
|
87
|
88
|
** |
103
|
104
|
105
|
106
|
107
|
108
|
109
|
110
|
111
|
112
|
113
|
114
|
115
|
116
|
117
|
118
|
| *Lanthanoids | * |
57
|
58
|
59
|
60
|
61
|
62
|
63
|
64
|
65
|
66
|
67
|
68
|
69
|
70
|
|||
| **Actinoids | ** |
89
|
90
|
91
|
92
|
93
|
94
|
95
|
96
|
97
|
98
|
99
|
100
|
101
|
102
|
|||