Chromium - 24Cr: the essentials
- Name: chromium
- Symbol: Cr
- Atomic number: 24
- Relative atomic mass (Ar): 51.9961 (6)
- Standard state: solid at 298 K
- Appearance: silvery metallic
- Classification: Metallic
- Group in periodic table: 6
- Group name: (none)
- Period in periodic table: 4
- Block in periodic table: d
- Shell structure: 2.8.13.1
- CAS Registry: 7440-47-3
Chromium atoms have 24 electrons and the shell structure is 2.8.13.1. The ground state electronic configuration of neutral chromium is [Ar].3d5.4s1 and the term symbol of chromium is 7S3.
Chromium: description
Chromium is steel-gray, lustrous, hard, metallic, and takes a high polish. Its compounds are toxic. It is found as chromite ore. Siberian red lead (crocoite, PrCrO4) is a chromium ore prized as a red pigment for oil paints.
Emerald is a form of beryl (a beryllium aluminium silicate) which is green because of the inclusion of a little chromium into the beryl crytal lattice in place of some of the aluminium ions. Similarly, traces of chromium incorporated into the crystal lattice of corundum (crystalline aluminium oxide, Al2O3) as a replacement for some of the Al3+ ions results in another highly coloured gem stone, in this case the red ruby.

This sample is from The Elements Collection, an attractive and safely packaged collection of the 92 naturally occurring elements that is available for sale.

Cartoon by Nick D Kim ([Science and Ink], used by permission).
Chromium: physical properties
Density of solid: 7140 kg m-3
Molar volume: 7.23 cm3
Thermal conductivity: 94 W m‑1 K‑1
Chromium: heat properties
Melting point: 2180 [1907 °C (3465 °F)] K
Boiling point: 2944 [2671 °C (4840 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Chromium: atom sizes
Atomic radius (empirical): 140 pm
Molecular single bond covalent radius: 122 (coordination number 6) ppm
van der Waals radius: 245 ppm
Chromium: electronegativities
Pauling electronegativity: 1.66 (Pauling units)
Allred Rochow electronegativity: 1.56 (Pauling units)
Mulliken-Jaffe electronegativity: (no data)
Chromium: orbital properties
First ionisation energy: 652.87 kJ mol‑1
Second ionisation energy: 1590.69 kJ mol‑1
Third ionisation energy: 2987.1 kJ mol‑1
Chromium: abundances
Universe: 15000 ppb by weight
Crustal rocks: 140000 ppb by weight
Human: 30 ppb by weight
Chromium: crystal structure
Chromium: biological data
Human abundance by weight: 30 ppb by weight
Chromium is an essential trace element and has a role in glucose metabolism. It seems to have an effect in the action of insulin. In anything other than trace amounts, chromium compounds should be regarded as highly toxic.
Chromium: uses
Chromium: reactions
Reactions of chromium as the element with air, water, halogens, acids, and bases where known.
Chromium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of chromium where known.
Chromium: compound properties
Bond strengths; lattice energies of chromium halides, hydrides, oxides (where known); and reduction potentials where known.
Chromium: history
Chromium was discovered by Louis-Nicholas Vauquelin in 1797 at France. Origin of name: from the Greek word "chroma" meaning "colour", named for the many coloured compounds known for chromium..Chromium: isotopes
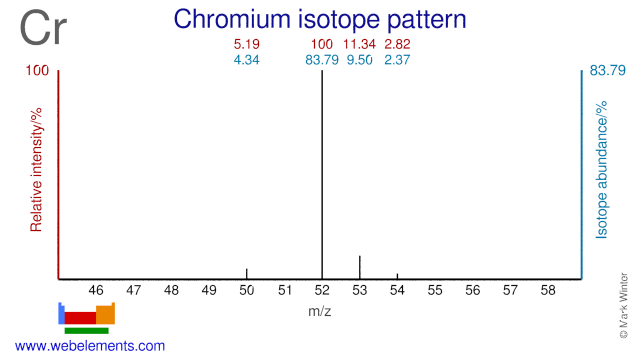
Several Chromium isotopes are used for medical applications. Cr-50 is used for the production of the radioisotope Cr-51 which is used for measuring blood volume and red blood cell survival. Cr-53 and Cr-54 are used for the study of chromium metabolism and studies into (adult) diabetes.
Chromium: isolation
Isolation: it is not normally necessary to make chromium in the laboratory as it is so readily available commercially. The most useful source of chromium commercially is the ore chromite, FeCr2O4. Oxidation of this ore by air in molten alkali gives sodium chromate, Na2CrO4 in which the chromium is in the +6 oxidation state. This is converted to the Cr(III) oxide Cr2O3 by extraction into water, precipitation, and reduction with carbon. The oxide is then further reduced with aluminium or silicon to form chromium metal.
Cr2O3 + 2Al → 2Cr + Al2O3
2Cr2O3 + 3Si → 4Cr + 3SiO2
Another kind of isolation is by electroplating processes. This involves the dissolution of Cr2O3 in sulphuric acid to give an electrolyte used for chromium electroplating.