Bromine - 35Br: the essentials
- Name: bromine
- Symbol: Br
- Atomic number: 35
- Relative atomic mass (Ar): 79.904 range: [79.901, 79.907]
- Standard state: liquid at 298 K
- Appearance: red-brown, metallic lustre when solid
- Classification: Non-metallic
- Group in periodic table: 17
- Group name: Halogen
- Period in periodic table: 4
- Block in periodic table: p
- Shell structure: 2.8.18.7
- CAS Registry: 7726-95-6
Bromine atoms have 35 electrons and the shell structure is 2.8.18.7. The ground state electronic configuration of neutral bromine is [Ar].3d10.4s2.4p5 and the term symbol of bromine is 2P3/2.
Bromine: description
Bromine is the only liquid nonmetallic element. It is a member of the halogen group. It is a heavy, volatile, mobile, dangerous reddish-brown liquid. The red vapour has a strong unpleasant odour and the vapour irritates the eyes and throat. It is a bleaching. When spilled on the skin it produces painful sores. It is a serious health hazard, and maximum safety precautions should be taken when handling it.
Cartoon by Nick D Kim ([Science and Ink], used by permission).
Bromine: physical properties
Density of solid: 4050 kg m-3
Molar volume: 19.78 cm3
Thermal conductivity: 0.12 W m‑1 K‑1
Bromine: heat properties
Melting point: 265.8 [‑7.3 °C (19 °F)] K
Boiling point: 332 [59 °C (138 °F)] K
Enthalpy of fusion: 20.5 kJ mol-1
Bromine: atom sizes
Atomic radius (empirical): 115 pm
Molecular single bond covalent radius: 114 (coordination number 1) ppm
van der Waals radius: 186 ppm
Bromine: electronegativities
Pauling electronegativity: 2.96 (Pauling units)
Allred Rochow electronegativity: 2.74 (Pauling units)
Mulliken-Jaffe electronegativity: 2.95 (14.3% s orbital)
Bromine: orbital properties
First ionisation energy: 1139.86 kJ mol‑1
Second ionisation energy: 2083.2 kJ mol‑1
Third ionisation energy: 3364.5 kJ mol‑1
Bromine: abundances
Universe: 7 ppb by weight
Crustal rocks: 3000 ppb by weight
Human: 2900 ppb by weight
Bromine: crystal structure
Bromine: biological data
Human abundance by weight: 2900 ppb by weight
Bromine may be an essential trace element for red algae and possibly mammals. It is found in the mollusc pigment "royal purple", although its role is not understood. Excessive bromide intake leads to depression and of weight loss.
Bromine: uses
Bromine: reactions
Reactions of bromine as the element with air, water, halogens, acids, and bases where known.
Bromine: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of bromine where known.
Bromine: compound properties
Bond strengths; lattice energies of bromine halides, hydrides, oxides (where known); and reduction potentials where known.
Bromine: history
Bromine was discovered by Antoine-J. Balard in 1826 at France. Origin of name: from the Greek word "bromos" meaning "stench".Bromine: isotopes
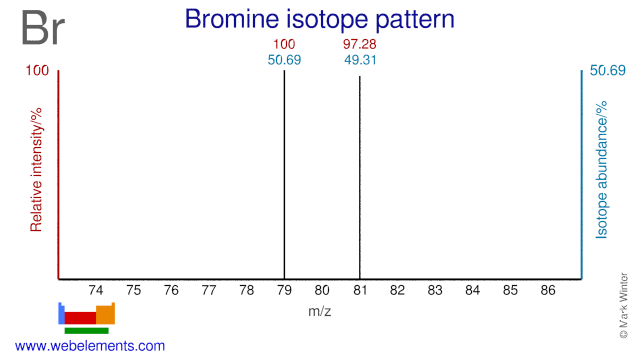
Both Bromine isotopes are used in nuclear medicine. Br-81 is used for the production of the radioisotope Kr-81m which is used for diagnostics. Br-79 can be used for the cyclotron production of Kr-77 which decays to the radioisotope Br-77 although the most common production route for Br-77 is via Se-77. Br-77 has been suggested for radiotherapy because of it electron capture decay and ease of labeling.
Bromine: isolation
Isolation: bromine is available commercially so it is not normally necessary to make it in the laboratory. Bromine also occurs in seawater as the sodium salt but in much smaller quantities than chloride. It is recovered commercially through the treatment of seawater with chlorine gas and flushing through with air. In this treatment, bromide is oxidized to bromine by the chlorine gas. The principle of oxidation of bromide to bromine is shown by the addition of a little chlorine water to aqueous solutions of bromide. These become brown as elemental bromine forms.
2Br- + Cl2 → 2Cl- + Br2
Small amounts of bromine can also be made through the reaction of solid sodium bromide, NaBr, with concentrated sulphuric acid, H2SO4. The first stage is formation of HBr, which is a gas, but under the reaction conditions some of the HBr is oxidized by further H2SO4 to form bromine and sulphur dioxide. This reaction does not work with the corresponding chlorides and fluorides.
NaBr (s) + H2SO4 (l) → HBr (g) + NaHSO4 (s)
2HBr (g) + H2SO4 (l) → Br2 (g) + SO2 (g) + 2H2O (l)